Artificial intelligence (AI) describes the ability of machines to perform tasks that are classified as intelligent. AI can be classified into two main categories: symbolic AI and machine learning (ML). Symbolic AI involves structuring an algorithm in a way that is easily understandable to humans. Machine learning (ML) is the predominant paradigm in the field of AI. The advent of artificial intelligence (AI) in medicine has transformed various medical specialties, including orthodontics. AI has shown promising results in enhancing the accuracy of diagnoses, treatment planning, and predicting treatment outcomes. Its usage in orthodontic practices worldwide has increased with the availability of various AI applications and tools.
- AI
- cephalometric analysis
- orthodontic
- dental diagnostics
- CBCT
1. Introduction
2. Dental Diagnostics
The use of medical imaging methods is essential in dental patient care because they aid in the clinical diagnosis of pathologies related to teeth and their surrounding structures [17][18][19][30,31,32]. Radiological methods, such as orthopantomograms (OPGs) and cone-beam computed tomography (CBCT), play crucial roles in orthodontic diagnosis, treatment planning, and monitoring [20][21][22][33,34,35]. However, with the increasing number of radiological examinations being performed [23][36], there is a need for a comprehensive tool to support the process of radiological diagnosis. In response to this demand, multimodular diagnostic systems based on AI have emerged. One such AI-based system, developed by Diagnocat Ltd. (San Francisco, CA, USA), utilizes CNNs and provides precise and comprehensive dental diagnostics. The system enables tooth segmentation and enumeration, oral pathology diagnosis (including periapical lesions and caries), and volumetric assessment. Several scientific papers have validated the diagnostic performance of this program, demonstrating its high efficacy and accuracy [24][25][26][27][28][37,38,39,40,41]. A study by Orhan et al. [24][37] reported that the AI system achieved 92.8% accuracy in the detection of periapical lesions in CBCT images and showed no statistically significant difference in volumetric measurements compared to manual methods. Similarly, a study evaluating the diagnostic accuracy of the program for periapical lesion detection on periapical radiographs (PRs) yielded comparable results [25][38]. However, conflicting results have also been reported, particularly regarding the accuracy of AI in the assessment of periapical lesions in OPGs [29][42]. In a recent study by Ezhov (2021) [30][43], the overall diagnostic performance of two groups, one aided by AI and the other unaided, was compared in oral CBCT evaluation. The AI system used in this researchtudy included modules for tooth and jaw segmentation, tooth localization and enumeration, periodontitis, caries, and periapical lesion detection. The results showed that the AI system significantly improved the diagnostic capabilities of dentists, with higher sensitivity and specificity values observed in the AI-aided group than in the unaided group (sensitivity: 0.8537 vs. 0.7672; specificity: 0.9672 vs. 0.9616). Several systematic reviews and meta-analyses have been conducted on the utilization of AI for identifying caries and periapical lucencies [31][32][33][34][35][36][37][38][39][40][41][42][44,45,46,47,48,49,50,51,52,53,54,55]. In a recent comprehensive study by Rahimi [41][54], the accuracy of classification models for caries detection was evaluated across 48 studies. The reported diagnostic accuracy varied significantly based on the imaging modality, ranging from 68% to 99.2%. The diagnostic odds ratio, which indicates the effectiveness of the test, also varied greatly from 2.27 to 32,767 across studies. The study concluded that deep learning models show promise for caries detection and may aid clinical workflows. One of the earliest meta-analyses conducted in 2019 on the computer-aided detection of radiolucent lesions in the maxillofacial region [33][46] yielded a pooled accuracy estimate of 88.75% (95% CI = 85.19–92.30); however, only four studies were included. A more recent meta-analysis by Sadr [39][52] included 18 studies and revealed that the pooled sensitivity and specificity were 0.925 (95% CI, 0.862–0.960) and 0.852 (95% CI, 0.810–0.885), respectively. The researcheuthors concluded that deep learning showed highly accurate results in detecting periapical radiolucent lesions in dental radiographs. These findings suggest that multimodal AI programs may serve as first-line diagnostic aids and decision support systems, improving patient care at multiple levels. Figure 1 shows a sample of the Diagnocat report.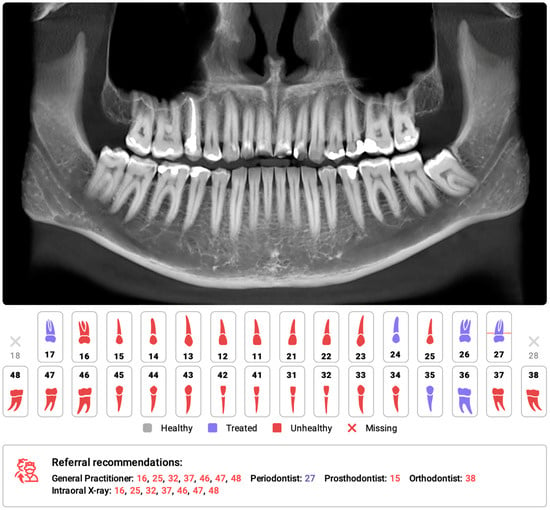
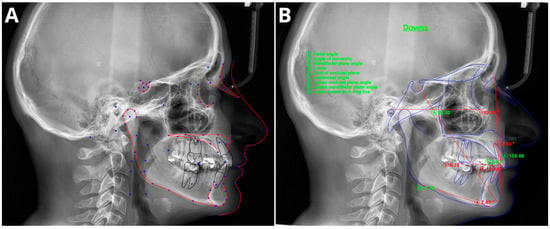
3. Cephalometric Analysis
Cephalometric analysis (CA) is an important diagnostic tool in orthodontics that has been in use since 1931 [43][56]. Over the years, advancements in technology have revolutionized CA by replacing manual assessments with digital software. This approach simplifies the measurement process and provides an automatic display of the analysis results. Automated CA has been shown to be more stable and repeatable than manual analyses, which rely heavily on operator-dependent landmark identification and often exhibit significant variability [44][45][46][47][57,58,59,60]. Accurate and repeatable landmark identification is crucial for reliable CA outcomes. Several studies have demonstrated the effectiveness of AI in identifying cephalometric landmarks. Although lateral radiography remains the most commonly used method in CA, recent AI advancements have sparked renewed interest in the use of cone-beam computed tomography (CBCT) [48][61]. The effectiveness of AI in identifying cephalometric landmarks has been studied since 1998 [49][62]. Numerous studies have used various automated methods and have consistently achieved high accuracy in landmark identification [46][47][50][51][52][53][54][55][56][57][58][59][59,60,63,64,65,66,67,68,69,70,71,72]. A recent study by Hwang et al. (2020) [47][60] concluded that automated cephalometric landmark identification can be as reliable as an experienced human reader. Similarly, Kim et al. [52][65], Lee et al. [58][71], and Dobratulin et al. [50][63] achieved landmark definition accuracies between 88% and 92% using AI. These researcheuthors also found that, compared with manual methods, AI methods demonstrated greater accuracy in landmark identification and reduced the time and human labor required. In other studies conducted by Hwang et al. [46][59] and Yu et al. [57][70], the researcheuthors found no statistically significant differences between the results of automated cephalometric analysis and those calculated via manually identified landmarks. Additionally, AI has been shown to significantly improve the workflow of practices, reducing analysis time by up to 80 times compared to manual analysis [59][72]. Figure 2 shows the definitions of the sampled cephalometric landmarks.