Antibiotics are used widely in human medicine, veterinary medicine, and agriculture. However, a portion of these compounds is excreted by treated organisms, entering water bodies through hospital effluents, domestic sewage, and agricultural waste. Furthermore, pharmaceutical production and improper disposal contribute to environmental contamination. The presence of antibiotics in the environment can have highly adverse consequences, such as the development and dissemination of bacterial resistance, reducing the effectiveness of antibiotics in treating infections. Several techniques are available for treating antibiotic contamination in water, including physical, chemical, and biological processes. The variety of techniques allows adaptation to the specific conditions of each case, selecting the most effective and cost-effective method considering the available infrastructure.
- environmental remediation
- emerging pollutants
- advanced materials
1. Introduction
2. Antibiotic Classes and Negative Effects on the Environment
Antibiotics are crucial in combating bacterial infections and are categorized based on their mechanisms and specificities [26][21]. Penicillin and cephalosporins are effective against Gram-positive and some Gram-negative bacteria, while macrolides treat respiratory and soft tissue infections [27][22]. With a broad spectrum of action, tetracyclines are effective against various bacteria but pose risks like photosensitivity and bacterial resistance [26][21]. Quinolones, such as ciprofloxacin, are potent but restricted due to safety concerns, while sulfonamides and glycopeptides are used for specific infections [28][23]. The indiscriminate use and inadequate disposal of antibiotics contribute to environmental pollution, fostering bacterial resistance and posing a significant global health challenge [7]. In addition, antibiotic residues disrupt ecosystems, impacting wildlife health and facilitating the horizontal transfer of resistance genes [11]. The environmental impact extends to non-target organisms [11], affecting aquatic life [29][24] and soil health, necessitating responsible antibiotic use and effective disposal strategies to minimize these consequences [30][25]. Figure 1 shows possible transformation and migration pathways for the antibiotics in the water environment and soil. Thus, it is vital to adopt responsible antibiotic use practices in both human and veterinary medicine. In this sense, effective disposal strategies, such as the appropriate treatment of effluents and improving techniques for remediating antibiotics in the ecosystem, are essential to minimize the environmental impact. Therefore, the following topic addresses and discusses the leading technologies for remediating antibiotics in the environment.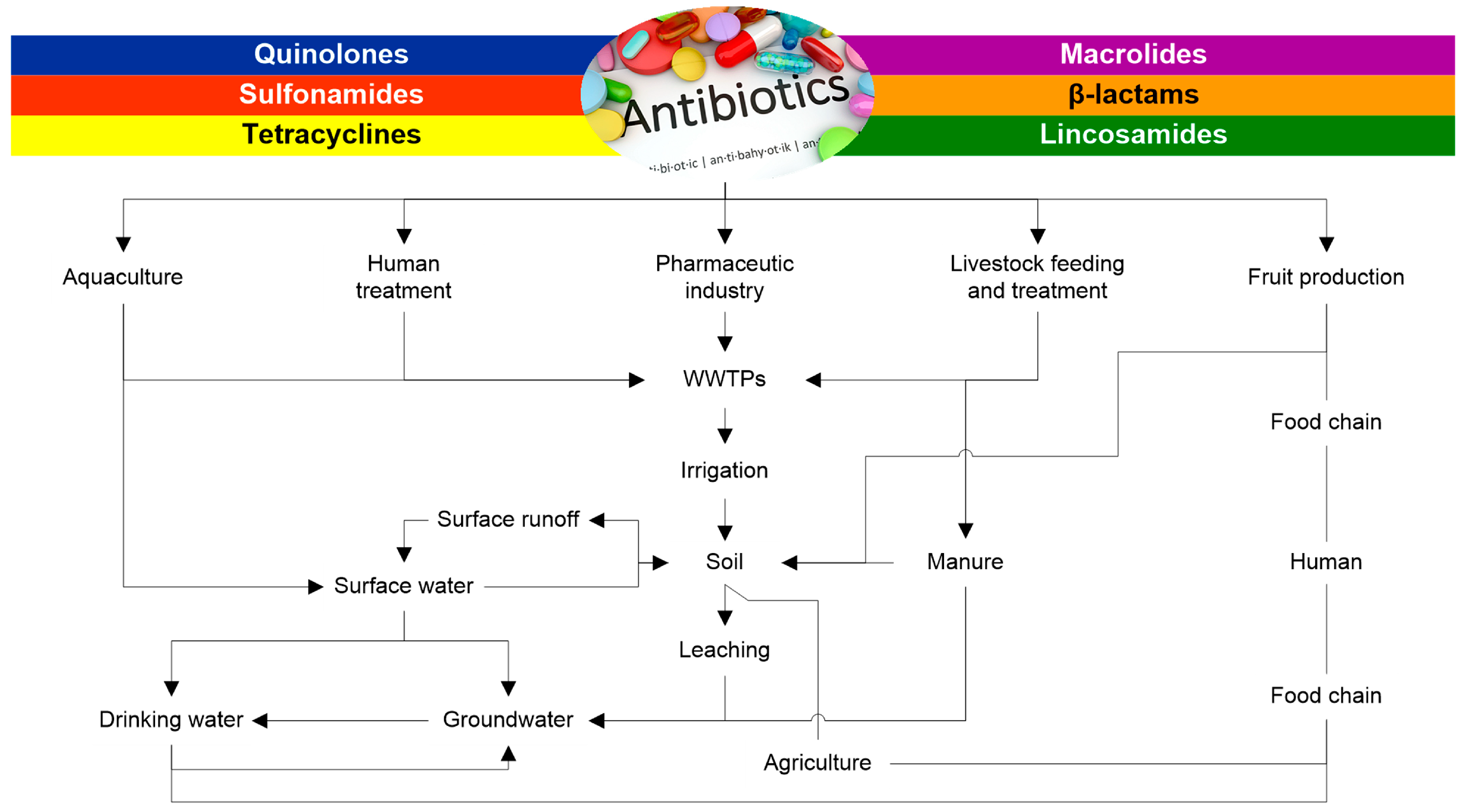
3. Antibiotics Remediation Technologies

3.1. Physical Techniques
3.2. Chemical Techniques
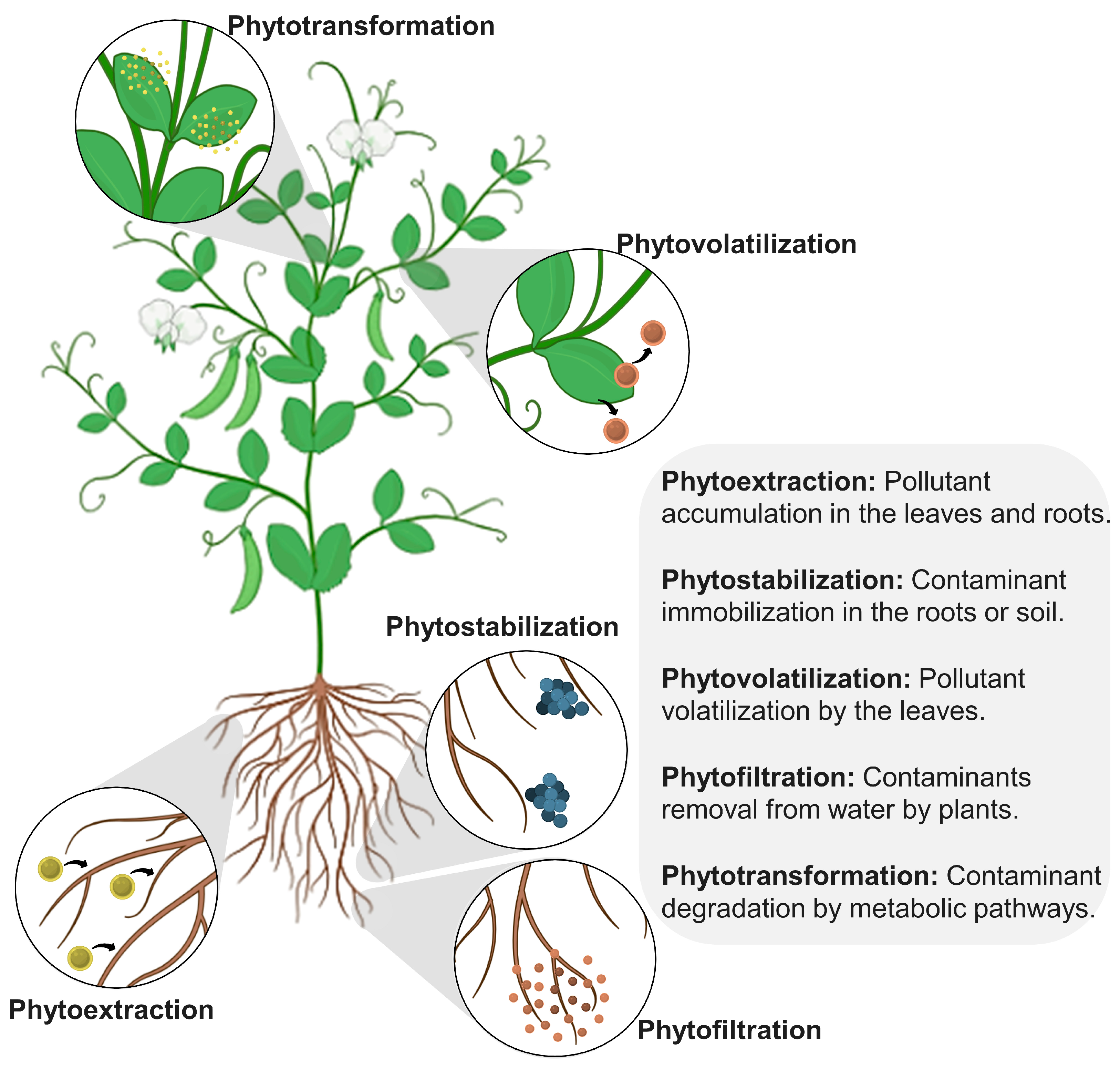
3.3. Biological Techniques
|
Technique |
Process |
Drug |
Removal |
Ref. |
||
|---|---|---|---|---|---|---|
|
Physical |
Adsorption |
Tetracycline |
99% |
|||
|
Adsorption |
Ciprofloxacin |
100% |
||||
|
Filtration and adsorption |
Tetracycline |
>90% |
||||
|
CeMnO3 |
Tetracycline hydrochloride |
[55][ | ||||
|
Chemical |
Electron beam | 49] |
||||
Sulfathiazole |
90% |
CaFe2O4 and LaFeO3 |
Tetracycline |
|||
|
Photocatalysis (Visible light) |
Amoxicillin, azithromycin, cefixime, and ciprofloxacin |
References
- de Oliveira, C.R.S.; da Silva Júnior, A.H.; Mulinari, J.; Ferreira, A.J.S.; da Silva, A. Fibrous Microplastics Released from Textiles: Occurrence, Fate, and Remediation Strategies. J. Contam. Hydrol. 2023, 256, 104169.
- Pham, D.N.; Clark, L.; Li, M. Microplastics as Hubs Enriching Antibiotic-Resistant Bacteria and Pathogens in Municipal Activated Sludge. J. Hazard. Mater. Lett. 2021, 2, 100014.
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater Treatment: A Short Assessment on Available Techniques. Alex. Eng. J. 2023, 76, 505–516.
- da Silva Júnior, A.H.; Mulinari, J.; de Oliveira, P.V.; de Oliveira, C.R.S.; Reichert Júnior, F.W. Impacts of Metallic Nanoparticles Application on the Agricultural Soils Microbiota. J. Hazard. Mater. Adv. 2022, 7, 100103.
- Huang, X.; Wen, D.; Wang, J. Radiation-Induced Degradation of Sulfonamide and Quinolone Antibiotics: A Brief Review. Radiat. Phys. Chem. 2024, 215, 111373.
- de Oliveira, C.R.S.; da Silva Júnior, A.H.; Mulinari, J.; Immich, A.P.S. Textile Re-Engineering: Eco-Responsible Solutions for a More Sustainable Industry. Sustain. Prod. Consum. 2021, 28, 1232–1248.
- Junaid, M.; Zainab, S.M.; Xu, N.; Sadaf, M.; Malik, R.N.; Wang, J. Antibiotics and Antibiotic Resistant Genes in Urban Aquifers. Curr. Opin. Environ. Sci. Health 2022, 26, 100324.
- Larsson, D.G.J.; Flach, C.-F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2022, 20, 257–269.
- da Silva Júnior, A.H.; de Oliveira, C.R.S.; Leal, T.W.; Mapossa, A.B.; Fiates, J.; Ulson de Souza, A.A.; Ulson de Souza, S.M.d.A.G.; da Silva, A. Organochlorine Pesticides Remediation Techniques: Technological Perspective and Opportunities. J. Hazard. Mater. Lett. 2024, 5, 100098.
- de la Fuente-Nunez, C.; Cesaro, A.; Hancock, R.E.W. Antibiotic Failure: Beyond Antimicrobial Resistance. Drug Resist. Updat. 2023, 71, 101012.
- Song, L.; Yang, S.; Gong, Z.; Wang, J.; Shi, X.; Wang, Y.; Zhang, R.; Wu, Y.; Wager, Y.Z. Antibiotics and Antibiotic-Resistant Genes in Municipal Solid Waste Landfills: Current Situation and Perspective. Curr. Opin. Environ. Sci. Health 2023, 31, 100421.
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766.
- Yan, F.; An, L.; Xu, X.; Du, W.; Dai, R. A Review of Antibiotics in Surface Water and Their Removal by Advanced Electrocoagulation Technologies. Sci. Total Environ. 2024, 906, 167737.
- Stylianou, M.; Christou, A.; Michael, C.; Agapiou, A.; Papanastasiou, P.; Fatta-Kassinos, D. Adsorption and Removal of Seven Antibiotic Compounds Present in Water with the Use of Biochar Derived from the Pyrolysis of Organic Waste Feedstocks. J. Environ. Chem. Eng. 2021, 9, 105868.
- Choi, K.-J.; Kim, S.-G.; Kim, S.-H. Removal of Antibiotics by Coagulation and Granular Activated Carbon Filtration. J. Hazard. Mater. 2008, 151, 38–43.
- Amaly, N.; EL-Moghazy, A.Y.; Nitin, N.; Sun, G.; Pandey, P.K. Design, Preparation, and Application of Novel Multilayer Metal-Polyphenol Composite on Macroporous Framework Melamine Foam for Effective Filtration Removal of Tetracycline in Fluidic Systems. Sep. Purif. Technol. 2023, 321, 124238.
- Kontogiannis, A.; Evgenidou, E.; Nannou, C.; Bikiaris, D.; Lambropoulou, D. MOF-Based Photocatalytic Degradation of the Antibiotic Lincomycin Enhanced by Hydrogen Peroxide and Persulfate: Kinetics, Elucidation of Transformation Products and Toxicity Assessment. J. Environ. Chem. Eng. 2022, 10, 108112.
- Anuar, N.F.; Iskandar Shah, D.R.S.; Ramli, F.F.; Md Zaini, M.S.; Mohammadi, N.A.; Mohamad Daud, A.R.; Syed-Hassan, S.S.A. The Removal of Antibiotics in Water by Chemically Modified Carbonaceous Adsorbents from Biomass: A Systematic Review. J. Clean. Prod. 2023, 401, 136725.
- Zheng, J.; Zhang, P.; Li, X.; Ge, L.; Niu, J. Insight into Typical Photo-Assisted AOPs for the Degradation of Antibiotic Micropollutants: Mechanisms and Research Gaps. Chemosphere 2023, 343, 140211.
- Bacha, A.-U.-R.; Nabi, I.; Chen, Y.; Li, Z.; Iqbal, A.; Liu, W.; Afridi, M.N.; Arifeen, A.; Jin, W.; Yang, L. Environmental Application of Perovskite Material for Organic Pollutant-Enriched Wastewater Treatment. Coord. Chem. Rev. 2023, 495, 215378.
- Bayan, E.M.; Pustovaya, L.E.; Volkova, M.G. Recent Advances in TiO2-Based Materials for Photocatalytic Degradation of Antibiotics in Aqueous Systems. Environ. Technol. Innov. 2021, 24, 101822.
- Zhu, T.; Su, Z.; Lai, W.; Zhang, Y.; Liu, Y. Insights into the Fate and Removal of Antibiotics and Antibiotic Resistance Genes Using Biological Wastewater Treatment Technology. Sci. Total Environ. 2021, 776, 145906.
- Guo, X.; Zhu, L.; Zhong, H.; Li, P.; Zhang, C.; Wei, D. Response of Antibiotic and Heavy Metal Resistance Genes to Tetracyclines and Copper in Substrate-Free Hydroponic Microcosms with Myriophyllum Aquaticum. J. Hazard. Mater. 2021, 413, 125444.
- Lima, É.; Oliveira, M.B.; Freitas, A. Antibiotics in Intensive Egg Production: Food Safety Tools to Ensure Regulatory Compliance. Food Chem. Adv. 2023, 3, 100548.
- Jia, W.-L.; Song, C.; He, L.-Y.; Wang, B.; Gao, F.-Z.; Zhang, M.; Ying, G.-G. Antibiotics in Soil and Water: Occurrence, Fate, and Risk. Curr. Opin. Environ. Sci. Health 2023, 32, 100437.
- Sharma, M.; Rajput, D.; Kumar, V.; Jatain, I.; Aminabhavi, T.M.; Mohanakrishna, G.; Kumar, R.; Dubey, K.K. Photocatalytic Degradation of Four Emerging Antibiotic Contaminants and Toxicity Assessment in Wastewater: A Comprehensive Study. Environ. Res. 2023, 231, 116132.
- Jin, Q.; Liu, W.; Dong, Y.; Lu, Y.; Yang, C.; Lin, H. Single Atom Catalysts for Degradation of Antibiotics from Aqueous Environments by Advanced Oxidation Processes: A Review. J. Clean. Prod. 2023, 423, 138688.
- Li, D.; Zhan, W.; Gao, X.; Wang, Q.; Li, L.; Zhang, J.; Cai, G.; Zuo, W.; Tian, Y. Aminated Waste Paper Membrane for Efficient and Rapid Filtration of Anionic Dyes and Antibiotics from Water. Chem. Eng. J. 2023, 455, 140641.
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of Natural Organic Matter in Drinking Water Treatment by Coagulation: A Comprehensive Review. Chemosphere 2018, 190, 54–71.
- Ba, S.; Haroune, L.; Soumano, L.; Bellenger, J.-P.; Jones, J.P.; Cabana, H. A Hybrid Bioreactor Based on Insolubilized Tyrosinase and Laccase Catalysis and Microfiltration Membrane Remove Pharmaceuticals from Wastewater. Chemosphere 2018, 201, 749–755.
- Mustafa, S.E.; Mustafa, S.; Abas, F.; Manap, M.Y.A.B.D.; Ismail, A.; Amid, M.; Elzen, S. Optimization of Culture Conditions of Soymilk for Equol Production by Bifidobacterium Breve 15700 and Bifidobacterium Longum BB536. Food Chem. 2019, 278, 767–772.
- Hamadeen, H.M.; Elkhatib, E.A. New Nanostructured Activated Biochar for Effective Removal of Antibiotic Ciprofloxacin from Wastewater: Adsorption Dynamics and Mechanisms. Environ. Res. 2022, 210, 112929.
- Xing, Z.-P.; Sun, D.-Z. Treatment of Antibiotic Fermentation Wastewater by Combined Polyferric Sulfate Coagulation, Fenton and Sedimentation Process. J. Hazard. Mater. 2009, 168, 1264–1268.
- Tang, X.; Fan, W.; Zhang, S.; Yan, B.; Zheng, H. The Improvement of Levofloxacin and Tetracycline Removal from Simulated Water by Thermosensitive Flocculant: Mechanisms and Simulation. Sep. Purif. Technol. 2023, 309, 123027.
- Zhu, Y.; Ma, J.; Zeng, S.; Li, X.; Lisak, G.; Chen, F. Advanced Treatment of Microplastics and Antibiotic-Containing Wastewater Using Integrated Modified Dissolved Air Flotation and Pulsed Cavitation-Impinging Stream Processes. J. Hazard. Mater. Adv. 2022, 7, 100139.
- Pattanayak, P.; Singh, P.; Bansal, N.K.; Paul, M.; Dixit, H.; Porwal, S.; Mishra, S.; Singh, T. Recent Progress in Perovskite Transition Metal Oxide-Based Photocatalyst and Photoelectrode Materials for Solar-Driven Water Splitting. J. Environ. Chem. Eng. 2022, 10, 108429.
- Piccirillo, G.; Moreira-Santos, M.; Válega, M.; Eusébio, M.E.S.; Silva, A.M.S.; Ribeiro, R.; Freitas, H.; Pereira, M.M.; Calvete, M.J.F. Supported Metalloporphyrins as Reusable Catalysts for the Degradation of Antibiotics: Synthesis, Characterization, Activity and Ecotoxicity Studies. Appl. Catal. B Environ. 2021, 282, 119556.
- Wang, X.; Jing, J.; Zhou, M.; Dewil, R. Recent Advances in H2O2-Based Advanced Oxidation Processes for Removal of Antibiotics from Wastewater. Chinese Chem. Lett. 2023, 34, 107621.
- Besegatto, S.V.; da Silva, A.; Campos, C.E.M.; de Souza, S.M.A.G.U.; de Souza, A.A.U.; González, S.Y.G. Perovskite-Based Ca-Ni-Fe Oxides for Azo Pollutants Fast Abatement through Dark Catalysis. Appl. Catal. B Environ. 2021, 284, 119747.
- Batool, S.; Shah, A.A.; Abu Bakar, A.F.; Maah, M.J.; Abu Bakar, N.K. Removal of Organochlorine Pesticides Using Zerovalent Iron Supported on Biochar Nanocomposite from Nephelium Lappaceum (Rambutan) Fruit Peel Waste. Chemosphere 2022, 289, 133011.
- Yin, S.; Wang, J.; Tong, Q.; Jiang, X.; Lu, P.; Zhu, Q.; Zhang, Q.; Zhang, Z.; Ueda, W. Degradation of Ciprofloxacin with Hydrogen Peroxide Catalyzed by Ironmolybdate-Based Zeolitic Octahedral Metal Oxide. Appl. Catal. A Gen. 2021, 626, 118375.
- Dutta, N.; Usman, M.; Ashraf, M.A.; Luo, G.; Zhang, S. Efficacy of Emerging Technologies in Addressing Reductive Dechlorination for Environmental Bioremediation: A Review. J. Hazard. Mater. Lett. 2022, 3, 100065.
- Li, W.; Liu, K.; Min, Z.; Li, J.; Zhang, M.; Korshin, G.V.; Han, J. Transformation of Macrolide Antibiotics during Chlorination Process: Kinetics, Degradation Products, and Comprehensive Toxicity Evaluation. Sci. Total Environ. 2023, 858, 159800.
- Lai, J.-H.; Dhenadhayalan, N.; Chauhan, A.; Chien, C.-W.; Yeh, J.-C.; Hung, P.-Q.; Lin, K.-C. Antibiotic Drugs Removal by Visible Light-Driven Photocatalysis Using Pt/Ru Nanoparticle-Decorated Hafnium Oxide Nanohybrids. J. Environ. Chem. Eng. 2022, 10, 108557.
- Zeng, Y.; Zhan, X.; Hong, B.; Xia, Y.; Ding, Y.; Cai, T.; Yin, K.; Wang, X.; Yang, L.; Luo, S. Surface Atom Rearrangement on Carbon Nitride for Enhanced Photocatalysis Degradation of Antibiotics under Visible Light. Chem. Eng. J. 2023, 452, 139434.
- Chen, C.; Bao, R.; Yang, L.; Tai, S.; Zhao, Y.; Wang, W.; Xia, J.; Li, H. Application of Inorganic Perovskite LaNiO3 Partial Substituted by Ce and Cu in Absorbance and Photocatalytic Degradation of Antibiotics. Appl. Surf. Sci. 2022, 579, 152026.
- Zhu, Z.; Wan, S.; Lu, Q.; Zhong, Q.; Zhao, Y.; Bu, Y. A Highly Efficient Perovskite Oxides Composite as a Functional Catalyst for Tetracycline Degradation. Sep. Purif. Technol. 2022, 281, 119893.
- Huy, B.T.; Nguyen, X.C.; Bui, V.K.H.; Tri, N.N.; Rabani, I.; Tran, N.H.T.; Ly, Q.V.; Truong, H.B. Photocatalytic Degradation of Antibiotic Sulfamethizole by Visible Light Activated Perovskite LaZnO3. J. Environ. Sci. 2023, 1–14.
- Anusha, H.S.; Yadav, S.; Tenzin, T.; Prabagar, J.S.; Anilkumar, K.M.; Kitirote, W.; Shivaraju, H.P. Improved CeMnO3 Perovskite Framework for Visible-Light-Aided Degradation of Tetracycline Hydrochloride Antibiotic Residue and Methylene Blue Dye. Int. J. Environ. Sci. Technol. 2023, 20, 13519–13534.
- Tuna, Ö.; Karadirek, Ş.; Simsek, E.B. Deposition of CaFe2O4 and LaFeO3 Perovskites on Polyurethane Filter: A New Photocatalytic Support for Flowthrough Degradation of Tetracycline Antibiotic. Environ. Res. 2022, 205, 112389.
- Montes-Hernandez, G.; Feugueur, L.; Vernier, C.; Van Driessche, A.E.S.; Renard, F. Efficient Removal of Antibiotics from Water via Aqueous Portlandite Carbonation. J. Water Process Eng. 2023, 51, 103466.
- Wang, Y.; Lin, N.; Xu, J.; Jiang, H.; Chen, R.; Zhang, X.; Liu, N. Construction of Microwave/PMS Combined Dual Responsive Perovskite-MXene System for Antibiotic Degradation: Synergistic Effects of Thermal and Non-Thermal. Appl. Surf. Sci. 2023, 639, 158263.
- Mamba, G.; Mafa, P.J.; Muthuraj, V.; Mashayekh-Salehi, A.; Royer, S.; Nkambule, T.I.T.; Rtimi, S. Heterogeneous Advanced Oxidation Processes over Stoichiometric ABO3 Perovskite Nanostructures. Mater. Today Nano 2022, 18, 100184.
- Gonca, S.; Özdemir, S.; Tekgül, A.; Gokhan Unlu, C.; Ocakoglu, K.; Dizge, N. Synthesis and Characterization of Perovskite Type of La1-XBaxMnO3 Nanoparticles with Investigation of Biological Activity. Adv. Powder Technol. 2022, 33, 103346.
- Yang, N.; Tian, Y.; Zhang, M.; Peng, X.; Li, F.; Li, J.; Li, Y.; Fan, B.; Wang, F.; Song, H. Photocatalyst-Enzyme Hybrid Systems for Light-Driven Biotransformation. Biotechnol. Adv. 2022, 54, 107808.
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of Pesticides from Water and Wastewater: Chemical, Physical and Biological Treatment Approaches. Environ. Technol. Innov. 2020, 19, 101026.
- Wang, Y.; Ning, W.; Han, M.; Gao, C.; Guo, W.; Chang, J.-S.; Ho, S.-H. Algae-Mediated Bioremediation of Ciprofloxacin through a Symbiotic Microalgae-Bacteria Consortium. Algal Res. 2023, 71, 103062.
- Leal, T.W.; Lourenço, L.A.; Scheibe, A.S.; de Souza, S.M.A.G.U.; de Souza, A.A.U. Textile Wastewater Treatment Using Low-Cost Adsorbent Aiming the Water Reuse in Dyeing Process. J. Environ. Chem. Eng. 2018, 6, 2705–2712.
- Nguyen, H.T.; Siddiqui, S.I.; Maeng, S.K.; Oh, S. Biological Detoxification of Oxytetracycline Using Achromobacter-Immobilized Bioremediation System. J. Water Process Eng. 2023, 52, 103491.
- Pillay, L.; Machete, F.; Hart, R. Exploring the Use of Phytoremediation and Sustainable Methods of Agriculture in Alleviating the Pollution in the UThongathi River Estuary. Environ. Challenges 2022, 9, 100633.
- Fu, T.; Du, L.; Wu, S.; Zhao, M.; Zheng, X.; Wang, Z.; Zhang, Y.; Fan, C.; Wang, W.; Ran, F.; et al. Synthesis and Application of Wetland Plant-Based Functional Materials for Aqueous Antibiotics Removal. Sci. Total Environ. 2024, 908, 168214.
- Kanwar, P.; Meena, U.; Thakur, I.S.; Srivastava, S. Heavy Metal Phytoremediation by the Novel Prospect of Microbes, Nanotechnology, and Genetic Engineering for Recovery and Rehabilitation of Landfill Site. Bioresour. Technol. Rep. 2023, 23, 101518.
- Tian, R.; Zhang, R.; Uddin, M.; Qiao, X.; Chen, J.; Gu, G. Uptake and Metabolism of Clarithromycin and Sulfadiazine in Lettuce. Environ. Pollut. 2019, 247, 1134–1142.
- Mulinari, J.; Junior, F.W.R.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; Scariot, M.A.; Radünz, L.L.; Mossi, A.J. Biochar as a Tool for the Remediation of Agricultural Soils. In Biochar and Its Application in Bioremediation; Springer Nature: Singapore, 2021; pp. 281–303.
- Miao, S.; Zhang, Y.; Men, C.; Mao, Y.; Zuo, J. A Combined Evaluation of the Characteristics and Antibiotic Resistance Induction Potential of Antibiotic Wastewater during the Treatment Process. J. Environ. Sci. 2024, 138, 626–636.
- Huang, K.; Yang, S.; Liu, X.; Zhu, C.; Qi, F.; Wang, K.; Wang, J.; Wang, Q.; Wang, T.; Ma, P. Adsorption of Antibiotics from Wastewater by Cabbage-Based N, P Co-Doped Mesoporous Carbon Materials. J. Clean. Prod. 2023, 391, 136174.
- Míguez-González, A.; Cela-Dablanca, R.; Barreiro, A.; Rodríguez-López, L.; Rodríguez-Seijo, A.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Castillo-Ramos, V.; Álvarez-Rodríguez, E. Adsorption of Antibiotics on Bio-Adsorbents Derived from the Forestry and Agro-Food Industries. Environ. Res. 2023, 233, 116360.
- Liu, N.; Huang, W.; Li, Z.; Shao, H.; Wu, M.; Lei, J.; Tang, L. Radiolytic Decomposition of Sulfonamide Antibiotics: Implications to the Kinetics, Mechanisms and Toxicity. Sep. Purif. Technol. 2018, 202, 259–265.
- Primožič, M.; Kravanja, G.; Knez, Ž.; Crnjac, A.; Leitgeb, M. Immobilized Laccase in the Form of (Magnetic) Cross-Linked Enzyme Aggregates for Sustainable Diclofenac (Bio)Degradation. J. Clean. Prod. 2020, 275, 124121.
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of Carbamazepine by Laccase-Mediator System: Kinetics, by-Products and Toxicity Assessment. Process Biochem. 2018, 67, 147–154.
- Touza-Otero, L.; Landin, M.; Diaz-Rodriguez, P. Fighting Antibiotic Resistance in the Local Management of Bovine Mastitis. Biomed. Pharmacother. 2024, 170, 115967.
- Kadkhodayan, H.; Alizadeh, T. Manufacturing Visible-Light-Driven Heterojunction Photocatalyst Based on MOFs/Bi2WZnTiO9 Triple Perovskite/Carbonous Materials for Efficient Removal of Poisons, Antibiotics, and Inorganic Pollutants. J. Phys. Chem. Solids 2023, 183, 111620.
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the Catalytic Activity of a Doped SrFeO3 for Water Pollutants Removal: Effect of Light and Temperature. Appl. Catal. B Environ. 2017, 207, 174–181.
- Hu, Z.; Yan, Q.; Wang, Y. Dynamic Surface Reconstruction of Perovskite Oxides in Oxygen Evolution Reaction and Its Impacts on Catalysis: A Critical Review. Mater. Today Chem. 2023, 34, 101800.
- Oliveira, L.; Venâncio, R.; de Azevedo, P.V.; Anchieta, C.G.; C. M. Nepel, T.; Rodella, C.B.; Zanin, H.; Doubek, G. Reviewing Perovskite Oxide Sites Influence on Electrocatalytic Reactions for High Energy Density Devices. J. Energy Chem. 2023, 81, 1–19.
