You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Stefano Priola and Version 3 by Jessie Wu.
Intracranial aneurysms represent a major global health burden. Rupture of an intracranial aneurysm is a catastrophic event. Without access to treatment, the fatality rate is 50% in the first 30 days. Over the last three decades, treatment approaches for intracranial aneurysms have changed dramatically. There have been improvements in the medical management of aneurysmal subarachnoid haemorrhage, and there has been an evolution of treatment strategies. Endovascular therapy is now the mainstay of the treatment of ruptured intracranial aneurysms based on robust randomised controlled trial data.
- endovascular coiling
- surgical clipping
- unruptured intracranial aneurysm
1. Introduction
Intracranial aneurysms (IA) are focal pathological dilatations of the cerebral arteries. While typically asymptomatic, the rupture of an IA leading to SAH is responsible for 5% of all strokes [1]. IA account for 80–85% of non-traumatic subarachnoid haemorrhage (SAH), but can also lead to intraparenchymal or subdural haemorrhage [1]. The average incidence of aneurysmal bleeding is approximately 9 per 100,000 person-years with the highest rates reported in Japanese and Finnish populations [2]. Though the annual incidence rates of ruptured IA are relatively stable, unruptured intracranial aneurysms (UIA), are increasingly detected in clinical practice, due to the widespread availability of advanced cross-sectional neuroimaging. The prevalence of IA is variable and depends on the evaluation methods used but IA are estimated to be found in 2–3.2% of the general population with a male to female ratio of 1:2 [3].
Aneurysmal subarachnoid haemorrhage is a catastrophic event with very high morbidity and mortality. About 15% of patients with ruptured IA could not even reach a hospital and there is a 50% thirty-day mortality in the remaining survivors [4]. Compared to other subtypes of stroke, patients affected due to SAH tend to be younger, which results in a greater loss of productive life [5]. Rebleeding is the most imminent danger; which drastically decreases the chances of a good outcome. The risk of rebleeding is at its maximum in the initial 24 h, during which the risk ranges from 4.1% to 17.3% [6]. Therefore, early diagnosis and aneurysm occlusion is a priority. Nowadays, many centres tend to operate within the first 24 h of onset via an endovascular or open surgical modality. On the other hand, unruptured aneurysms, with an annual risk of rupture 0.49–1.8%, represent a challenge in terms of conservative management versus aggressive intervention [7]. Further, selecting an appropriate treatment strategy is a complex decision, which varies according to patient and aneurysm-related factors.
2. Aneurysm Pathophysiology
The cerebrovascular system is inherently susceptible to aneurysm formation, although the aetiology of these abnormalities may be diverse. The important ones include the absence of an external elastic lamina, paucity of supportive perivascular tissues, and attenuated tunica media in the intracranial arterial system [8]. The distribution of cerebral aneurysms around the bifurcations or branch location points towards hemodynamic factors and/or wall shear stress as a principal determinant for aneurysm formation, growth and rupture (Figure 1A). Incidence of IA is high in females, smokers, hypertensives, and a few geographical regions. Apart from this, multiple familial conditions are associated with a high incidence of IA, such as autosomal dominant inherited polycystic kidney disease, Marfan syndrome, fibromuscular dysplasia (FMD), alpha1-antitrypsin deficiency, etc. (Table 1). The list is exhaustive and illustrative of the fact that multiple genetic, hemodynamic and environmental factors act together in the formation and growth of IA [8].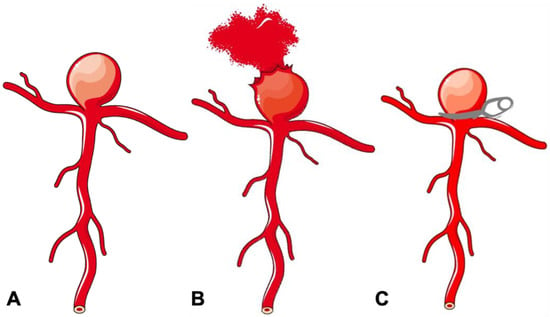
Figure 1.
Schematic diagram of a bifurcation aneurysm. (
A
) Bifurcation aneurysm. (
B
) Ruptured bifurcation aneurysm. (
C
) Surgical clipping of bifurcation aneurysm.
Table 1.
Risk factors for aneurysm formation.
| 1. Female Sex |
| 2. Smoking |
| 3. Hypertension |
| 4. Coarctation of the aorta |
| 5. Racial Predisposition e.g., Japanese, Finnish populations |
| 6. Hereditary syndromes e.g., Ehlers-Danlos syndrome, Pseudoxanthoma elasticum, Polycystic Kidney Disease, FMD |
| 7. Familial aneurysm |
3. Aneurysm Classification
IA can be classified using several schemes (Table 2). Clinically, they can be classified on the basis of rupture status: ruptured or unruptured. According to morphology, aneurysms are classified into saccular and non-saccular types. Non-saccular IA are further classified into fusiform, dolichoectatic, and dissecting aneurysms. According to location in the intracranial circulation, aneurysms are divided into anterior (90%) and posterior circulation (10%) aneurysms. IA are also classified by size into small (<10 mm), large (10–25 mm), and giant (>25 mm) categories. Aneurysm angioarchitecture is important for planning suitability for endovascular management and can thus be classified according to neck size and relationship with the dome. Apart from serving a descriptive purpose, these classification systems also help in predicting prognosis, plan management, and appropriate treatment strategies. For example, despite advances in knowledge about aneurysm pathophysiology and technology, aneurysms with a large size (>10 mm), wide neck, unfavourable dome-to-neck ratio (<2), posterior circulation location and fusiform configuration remain therapeutic challenges with >20% faring poorly despite the best endovascular or surgical treatment [9][10][9,10]. ReseaOurchers' review is focussed on the management of the saccular aneurysm, which is the single largest group of IA (Figure 1A).Table 2.
Aneurysm Classification.
| Classification | Description |
|---|---|
| Rupture Status | Unruptured, Ruptured |
| Shape | Saccular, Fusiform, Dissecting |
| Location | Anterior Circulation (Anterior Communicating Artery, Middle Cerebral Artery aneurysm, etc.), Posterior Circulation (Basilar Artery, Vertebral artery aneurysm, etc.) |
| Size | Small (<10 mm), Large (>10–25 mm), Giant (>25 mm) |
| Aetiology | Atherosclerotic, Mycotic, Traumatic, Congenital, Hemodynamic (Flow-related), Idiopathic |
4. The Evolution of Current Endovascular Treatment Options
While Egaz Moniz pioneered diagnostic cerebral angiography in 1927, the development of the endovascular approach to aneurysm treatment took several years to materialize [11][36]. In 1964, Luessenhop and Velasquez performed the first catheterization of intracranial vessels [12][37]. Following this, in 1974, the advent of the use of both detachable and non-detachable balloons was described by Serbinenko in his study involving 300 patients, marking the beginning of the balloon era in the endovascular treatment of intracranial aneurysms [13][38]. The rigid structure of the balloon produced an angioplasty of the aneurysm wall with consequent immediate or delayed aneurysm rupture. As a result, the use of balloons remained limited to the small clinical series of inoperable aneurysms. The initiation of the modern era of neuroendovascular therapy occurred with the development and utilization of electrolytically detachable platinum coils (GDC) in 1991 by Guglielmi et al. [14][39]. These coils were soft, retrievable, and detachable by the operator, which solved many of the problems with other earlier techniques. With continued advances in endovascular technology, aneurysm coiling quickly emerged as an accepted and viable alternative to surgical clipping over the last decade of the 20th century.
4.1. Aneurysm Coiling
Once approved by the US Food and Drug Administration (FDA) in 1995, aneurysm coiling became the primary treatment modality of IA in numerous centres [15][16][40,41]. The goal of coiling is to achieve dense packing and induce rapid coagulation within the aneurysm sac, thereby isolating it from active circulation. Aneurysm geometry is an important determinant of treatment decision and outcome. Unassisted coiling is typically appropriate for intracranial aneurysms (IA) with favourable anatomical characteristics such as neck width, dome-to-neck ratio, aspect ratio, and relationship with branch vessels. For more complex aneurysms exhibiting unfavourable anatomy, adjunct techniques, as outlined below, may be employed for treatment.
4.2. Balloon-Assisted Coiling (BAC)
In the treatment of complex, wide-neck aneurysms, it is difficult to achieve coil stability and dense packing with an unassisted coiling. Additional support is necessary in such situations to provide a scaffold. The technique of BAC was first described by Moret et al. in 1997 [17][42]. The procedure consists of the temporary inflation of a non-detachable balloon across the aneurysm neck during each coil placement (Figure 23A,B). It also stabilizes the microcatheter position during coiling and acts as a safety valve in the event of intra-operative rupture. Nowadays, a variety of balloons, including compliant, hypercompliant, round-shaped, and double-lumen balloons, are available. The added advantage with the use of a double-lumen balloon is that it has separate inflation and working lumens and allows the placement of a stent at the end of procedure if required.
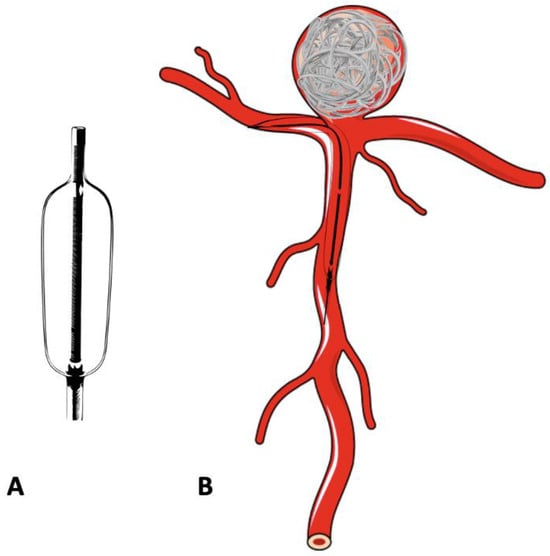

Figure 23. Schematic diagram of a double-lumen balloon and its use in the treatment of bifurcation aneurysm. (A) Double -lumen balloon. (B) Bifurcation aneurysm treated with balloon-assisted coiling.
4.3. Stent-Assisted Coiling
Though the balloon provides a necessary scaffold for coiling, it is temporary and removed at the end the end of the procedure. Hence BAC is inadequate in cases with extremely unfavourable aneurysmal anatomy. A stent which provides permanent support to coils overcomes this limitation at the cost of requiring long-term antiplatelet therapy to maintain the stent patency (Figure 34A and Figure 45B). In 1997, Higashida et al. demonstrated the use of stents in surgically inoperable posterior circulation aneurysms [18][43]. The initially used stents were balloon mounted. The first self-expanding stent designed specifically for intracranial aneurysm treatment was the Neuroform stent (Stryker Neurovascular, Fremont, CA, USA) which received FDA approval in 2002 [19][44]. Stents which are nowadays available are high porosity, self-expanding stents with a highly flexible configuration to permit navigation across tortuous vessels whilst minimising vascular intimal injury. Furthermore, apart from their use in side wall aneurysms, single or multiple stents are used in different configurations, such as a X, Y, T, waffle-cone, or shelf, to provide the necessary scaffold at the neck of the aneurysm, which may not be possible with BAC alone [20][21][45,46].

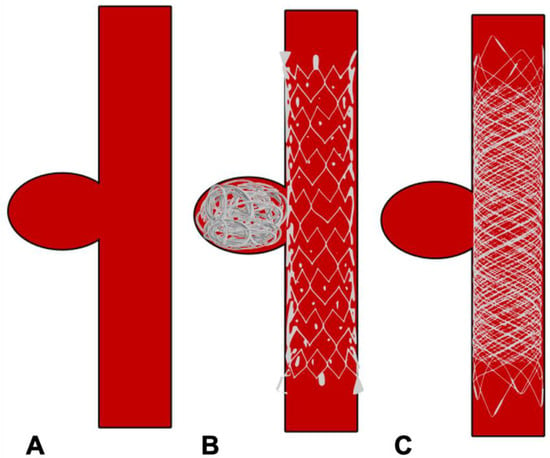

Figure 34. The difference between a conventional intracranial stent and flow diverter stent. (A) Conventional stent with higher porosity. (B) Flow diverter stent with reduced porosity.

Figure 45. Schematic diagram showing treatment of side wall aneurysm. (A) Side wall aneurysm (B) Endovascular treatment using a conventional stent and coils (C) Endovascular treatment using flow diverter stent without coils.
The most troublesome complications with BAC and SAC are the risks of thromboembolic events and procedural aneurysmal rupture. The multicentre CLARITY trial compared the safety and efficacy of the remodelling technique (BAC and/or SAC) with conventional coiling in ruptured aneurysms. The study revealed better immediate postoperative occlusion rates in the remodelling group than conventional coiling (94.9% vs. 88.7%) with improved packing density (39.3% vs. 36.7%) despite unfavourable anatomy in the remodelling group. The equivalent rate of treatment-related complications (remodelling group 16.9% vs. conventional coiling 17.4%) and treatment-related cumulative morbidity and mortality (remodelling group 3.8% vs. conventional coiling 5.1%) was noted in both of the groups. A meta-analysis by Wang et al., comparing BAC and SAC, demonstrated no difference between complete occlusion rates (COR) at the end of the procedure, rate of post-treatment complications, or rate of retreatment between groups. However, the COR at 6 months or later was better with SAC [22][47]. Piotin et al., found lower recurrence rates with SAC compared to conventional coiling [23][48]. The reason for improved outcomes with stent-assisted coiling may be explained by fluid dynamic studies, which revealed that stent placement causes a reduction of aneurysmal vortex speed and decreased interaction with parent vessel flow, depending on porosity [24][49]. This discovery subsequently led to the development of flow diverter stents.
4.4. Flow-Diverting Stents
Flow diverters (FD), working upon the principle of modifying intra-aneurysmal haemodynamic, are another substantial addition to the arsenal of aneurysm treatment. The design of flow-diverting stents is akin to conventional stents but with significantly lower porosity, serving two functions (Figure 34A,B). Firstly, it redirects blood flow away from the aneurysm into the parent vessel, promoting stasis and thereby natural thrombosis in the aneurysm (Figure 45). The thrombus acts like a scar which collapses over time. Secondly, FD provide a scaffold for endothelial growth, thereby isolating the aneurysm from the parent circulation [25][50]. By allowing neo-endothelisation to occur, FD heals the weakened abnormal arterial wall and therefore, provides a curative outcome as compared to other therapeutic options. Unlike surgical clipping and coil embolization which provide immediate protection against the future risk of rupture, the process of stasis, sac thrombosis and vessel healing by FD occurs slowly and can take up to 6 to 12 months after the treatment. However, compared to coiling, which has an overall recanalization rate of 20% and retreatment rate of up to 10%, very high (>90%) occlusion rates have been reported with FD on long-term follow-up [26][27][28][51,52,53].
The first FD introduced in the field of neurovascular intervention was the Pipeline Embolization Device (Medtronic Neurovascular, Irvine, CA, USA) in 2007, which received FDA approval in 2011. After its success and acceptance, a plethora of new devices have entered into the ever-growing field of FD. All of these devices work on the same haemodynamic principle, with some differences, mainly in terms of the number of wires, delivery system, and stent material used. Initial studies with FD focused on lesions such as giant, fusiform, difficult-to-treat aneurysms [29][30][54,55]. However, with increasing experience, there is an expanding list of indications for FD including complex wide-neck aneurysms, recanalized aneurysms, distal anterior circulation, and very small aneurysms which are otherwise challenging to treat by conventional endovascular options [31][32][33][56,57,58]. Though its use is precluded in acutely ruptured aneurysms due to the lack of immediate occlusion and need of antiplatelet therapy, a recently published meta-analysis (20 studies with 126 patients) on FD use in ruptured IA demonstrated good clinical outcomes (81%) with a complete occlusion rate of 90%. The majority (73%, 92/126) of ruptured aneurysms were treated with FD placement only [34][59]. Recently, the use of FD has been studied in middle cerebral artery (MCA) bifurcation aneurysms, with 68% occlusion at 6 months and 95% occlusion at 12 months along with 8.6% morbidity without any mortality [35][60]. So far there is insufficient evidence at present to support the routine use of FD in ruptured or in bifurcation aneurysms.
Complications associated with FD include thromboembolic events, bleeding risk associated with antiplatelet use, the risk of stent stenosis or thrombosis, and the risk of perforator occlusion inherent to the mechanism of action of FD. The International Retrospective Study of the Pipeline Embolization Device (IntrePED), in which 793 patients with 906 aneurysms were included, reported a 4.7% risk of ischemic stroke due to thromboembolic complications and a total of 8.4% for neurological morbidity and mortality [36][61]. Due to its endoluminal approach without accessing the aneurysm sac, FD placement reduces the possibility of intraprocedural aneurysm rupture inherent with conventional endosaccular coiling. However, they are associated with unique complications including distal intraparenchymal haemorrhage and delayed aneurysm rupture [37][62]. Though the exact aetiology for both of these complications is unknown, the postulated theory for intraparenchymal haemorrhage is that it may be a result of hemodynamic alterations secondary to device placement or the haemorrhagic conversion of microinfarcts. Delayed aneurysm rupture is probably due to inflammation related to intra-aneurysmal thrombus formation and/or hemodynamic changes in an unstable aneurysm induced by the placement of the device. In the IntrePED study, the rates of distal intraparenchymal haemorrhage and spontaneous rupture were about 2% and 0.5%, respectively. In the study, the lowest complication rates were seen when it was used to treat small ICA aneurysms, whilst the highest complications rates were noted with posterior circulation and giant aneurysms [36][61]. Hoe et al. reported the highest rate of delayed aneurysm rupture in giant, symptomatic aneurysms with a high aspect ratio, associated with morbidity and mortality rates of >70% [38][63]. Nevertheless, FD are a viable and effective tool for the treatment of complex aneurysms with high occlusion rates and a good safety profile.
