Children with epilepsy are affected by several factors, including clinical and social variables. Among these variables, cognitive decline and behavioral disturbances, perceptions of stigma, and fatigue can lead to reductions in quality of life (QOL). Epileptic activities, including seizure severity, frequent seizures, and status epilepticus (SE), have been identified as important predictors of QOL. In addition, the frequency of interictal epileptiform discharges (IEDs) on electroencephalogram (EEG) may also be an important predictor of QOL, because IEDs can lead to cognitive decline and behavioral disturbances. Moreover, frequent seizures and/or IEDs may play a role in emotional mediators, such as stigma and fatigue, in childhood epilepsy. Seizure severity and/or IEDs are, therefore, important QOL-related factors in childhood epilepsy.
- stigma
- seizure severity
- interictal epileptiform discharge (IED)
- quality of life (QOL)
- behavior
- cognition
- frontal
1. Introduction
2. Seizure Severity in Childhood Epilepsy
2.1. Seizure Severity and Cognitive/Behavioral Disturbances
Neuropsychological impairments, such as cognitive decline and behavioral disturbances, reduce QOL in childhood epilepsy and may be induced by frontal lobe dysfunction. In addition, QOL reduction in children can also reduce QOL in the family. Thus, frontal lobe dysfunction can result in reduced QOL for both the child and their family. The frontal lobes are the largest cortical regions of the brain, comprising approximately 40% of the cerebral cortex. Among these regions, the prefrontal regions involve wide networks [14]. Because of these connections, the prefrontal cortex can receive abundant information from all parts of the cerebrum and can affect information processing in those parts. Prefrontal lobe neurons and glial cells are readily influenced by various factors, so prefrontal lobe functions are regarded as being vulnerable for a long period [15]. Accordingly, severe seizures, such as frequent or prolonged seizures, result in negative effects on prefrontal lobe functions more easily than on other cortical regions [14,15][14][15]. Considering these findings, epilepsy associated with prefrontal regions in children may be associated with several neuropsychological impairments in comparison with healthy subjects [3]. In other cerebral regions, seizures can result in memory and learning disturbances, which relate to temporal lobe dysfunctions [16]. Temporal lobe seizures can also lead to behavioral disturbances [17]. However, direct relationships between seizures and temporal or other cerebral lobe functioning have not been fully revealed. Further research is needed to discuss these aspects.2.1.1. Prefrontal Lobe Growth in Frontal Lobe Epilepsy
Understanding how frontal lobe epilepsy (FLE) impacts the life of patients is important. In a serial three-dimensional (3D) magnetic resonance imaging (MRI) volumetric study, the growth of the frontal and prefrontal lobes in children with drug-responsive FLE without neuropsychological impairments was similar to that in healthy subjects [3]. In contrast, frontal and prefrontal lobe growth disturbances were present during the active epileptic phase in refractory FLE patients with cognitive decline and behavioral disturbances. However, a difference associated with the active seizure period was present. A short active seizure period was associated with prompt growth recovery. In children with a longer active seizure period, the growth disturbance was more severe, and growth recovery took longer [3] (Table 1). Frequent seizures in children with FLE may thus induce prefrontal lobe growth disturbances, which can lead to neuropsychological impairments.| Epileptic Syndrome | Findings |
|---|---|
| Frontal lobe epilepsy (FLE) | # Frontal/prefrontal lobe volumes and the prefrontal-to-frontal lobe volume ratio increased serially in the drug-responsive FLE patients without cognitive decline/behavioral disturbances and non-epilepsy children. # Frontal and prefrontal lobe growth disturbances were present during the active seizure period in the refractory FLE patients with cognitive decline and behavioral disturbances. # Active seizure period was short in children with prompt growth recovery. # The growth disturbance was more severe, and the growth recovery was required a long time in children with a longer active seizure period. |
| Self-limited epilepsy with centrotemporal spikes (SeLECTS) | # Frontal and prefrontal lobe growth disturbances were present during the active seizure period in patients presenting atypical evolution. # The growth disturbance was more severe, and the growth recovery was required a long time in a patient with a longer active seizure period. |
| Self-limited epilepsy with autonomic seizures (SeLEAS) | # Frontal and prefrontal lobe growth disturbances were present after episodes of SE in SeLEAS patients presenting with behavioral disturbances. # In a patient with only one episode of SE, growth disturbance soon recovered. # Conversely, recovery of growth ratios was delayed in patients with several episodes of SE. |
2.1.2. Prefrontal Lobe Growth in SeLECTS
SeLECTS is considered a condition free of neurological and psychological impairments. However, children with SeLECTS sometimes present with severe aggravation of epileptic manifestations, cognitive decline, and behavioral disturbances [18]. Frontal and prefrontal lobe volumes and, in particular, the prefrontal-to-frontal lobe volume ratio showed growth disturbances during the active seizure period in patients presenting with atypical evolution [14]. However, differences associated with the active seizure period were present. The active seizure period was shorter in patients with prompt growth recovery. In patients with a longer active seizure period, growth disturbances were more severe, and, again, growth recovery took longer in these patients [14] (Table 1). Seizure severity in SeLECTS may also be associated with prefrontal lobe growth disturbances, again leading to neuropsychological impairments.2.1.3. Prefrontal Lobe Growth in Self-Limited Epilepsy with Autonomic Seizures
Self-limited epilepsy with autonomic seizures (SeLEAS), which represents Panayiotopoulos syndrome, is generally accepted as lacking neuropsychological impairments. However, cognitive decline and behavioral disturbances may be present in at least some children with SeLEAS. SE can induce cerebral damage to various degrees. In SeLEAS patients, seizures tend to be prolonged, with subsequent focal or focal-to-bilateral tonic–clonic SE [4]. A sequential study using 3D–MRI volumetry showed that frontal and prefrontal lobe growth disturbances were present after episodes of SE in SeLEAS patients presenting with behavioral disturbances. In a patient with only one episode of SE, growth disturbances soon recovered. Conversely, the recovery of growth ratios was delayed in patients with several episodes of SE [4] (Table 1). Moreover, the cognitive scores, as measured using the Wechsler intelligence scale for children, dropped after the SE episodes [4]. The presence of SE in children with SeLEAS may thus induce growth disturbances in the prefrontal lobe, which can lead to neuropsychological impairments.2.2. QOL-Related Factors: Headache
Epilepsy and migraine are part of a heterogeneous family of neurological disorders [19]. The prevalence of headache is extremely high, so concomitant headache can be present in many patients with epilepsy. Approximately 35% of epileptic children experienced headaches in association with seizures in ourthe previous study [20]. The frequencies of seizures in children with and without seizure-associated headache were 4.1 and 1.3 times per year, respectively [20] (Table 2). Thus, seizure recurrence can induce headaches in association with seizures, which leads to reduced QOL in children with epilepsy.| QOL-Related Factors | Findings |
|---|---|
| Headache | # The frequency of seizures was 4.1 times per year in children with seizure-associated headache. # The frequency of seizures was 1.3 times per year in those with non-seizure-associated headache. # Frequent seizures may be in association with seizure-associated headache. |
| Fatigue | # The mean Fatigue Severity Scale scores of the children with epilepsy were significantly higher than those of the non-epilepsy children. # Frequency of seizures was sole significant clinical manifestation in association with fatigue. # A higher frequency of seizures was associated with more severe fatigue. |
| Stigma in children | # Children with frequent seizures presented psychosocial impairments in comparison with seizure-remission children. # Greater perceptions of stigma were in association with greater frequency of seizures. |
| Stigma in parents | # Parents of children with epilepsy showed significantly higher scores on the questionnaire than those of non-epilepsy children. # Greater perceptions of stigma were in association with frequency of seizures. |
2.3. QOL-Related Factors: Fatigue
Fatigue has a negative impact on QOL in patients with various chronic diseases, including epilepsy [21,22,23][21][22][23]. The mean Fatigue Severity Scale scores in epileptic children were significantly higher than those in non-epileptic children [24]. The frequency of seizures was identified as the only significant clinical manifestation associated with fatigue using multiple linear regression analysis. Moreover, children with frequent seizures presented with more severe fatigue [24] (Table 2). Accordingly, frequent seizures can lead to the presence of fatigue in children with epilepsy.2.4. QOL-Related Factors: Perception of Stigma among Children
The perception of stigma among epileptic patients is a negative psychological issue associated with a reduction in QOL. Stigma may be considered one of the psychological factors that affect QOL, along with seizure factors. Stigma has a negative effect on self-esteem and social status, thus leading to a poor prognosis, including isolation and delayed initiation of treatment for epileptic patients [25]. Frequent seizures could lead to psychosocial impairments in children. Thus, stigma has negative effects on social identity in children with epilepsy who experience frequent seizures.2.5. QOL-Related Factors: Perception of Stigma among Parents
Epilepsy in children can be a risk factor for stress in their parents [27,28,29,30][26][27][28][29]. Parents of children with intractable epilepsy tend to experience severe anxiety in relation to recurrent seizures, and this parental state of anxiety can lead to poor adaptive function in children [31][30]. Frequent seizures are, therefore, an important issue with respect to parenting stress [32][31]. The parents of children with epilepsy showed higher scores on the Parent Stigma Scale than the parents of healthy children [33][32]. Moreover, greater perceptions of stigma among parents correlated with higher seizure frequency [33][32] (Table 2). Accordingly, frequent seizures in children with epilepsy can lead to greater perceptions of stigma among parents. However, the relationship between seizures and stigma has only been analyzed individually without simultaneously considering other biopsychosocial factors; therefore, there may be limitations in the understanding of this relationship.3. The Treatment of Epilepsy in Children
3.1. Is the Urgent Suppression of Clinical Seizures Needed?
As mentioned above, the presence of frequent seizures and SE can induce growth disturbances in the prefrontal lobe, leading to neuropsychological impairments [3,4][3][4] (Figure 1). In addition, recovery from prefrontal lobe growth disturbances may depend on the active seizure period. In several epileptic syndromes, children with a shorter active seizure period can recover from disturbances in prefrontal lobe growth more rapidly. However, such recovery may be delayed in children with a longer active seizure period [3]. These findings support the hypothesized relationship between seizure activities and behavioral disturbances, i.e., “seizure activity per se disrupts behavior”, as suggested by Austin et al. [51][33]. In addition, SE in children can lead to prefrontal lobe growth disruption. Accordingly, SE can lead to neuropsychological impairments in association with prefrontal lobe growth disruptions (Figure 1). Another study indicated that damage to the frontal regions during childhood can cause deteriorations in neurobehavioral development [52][34].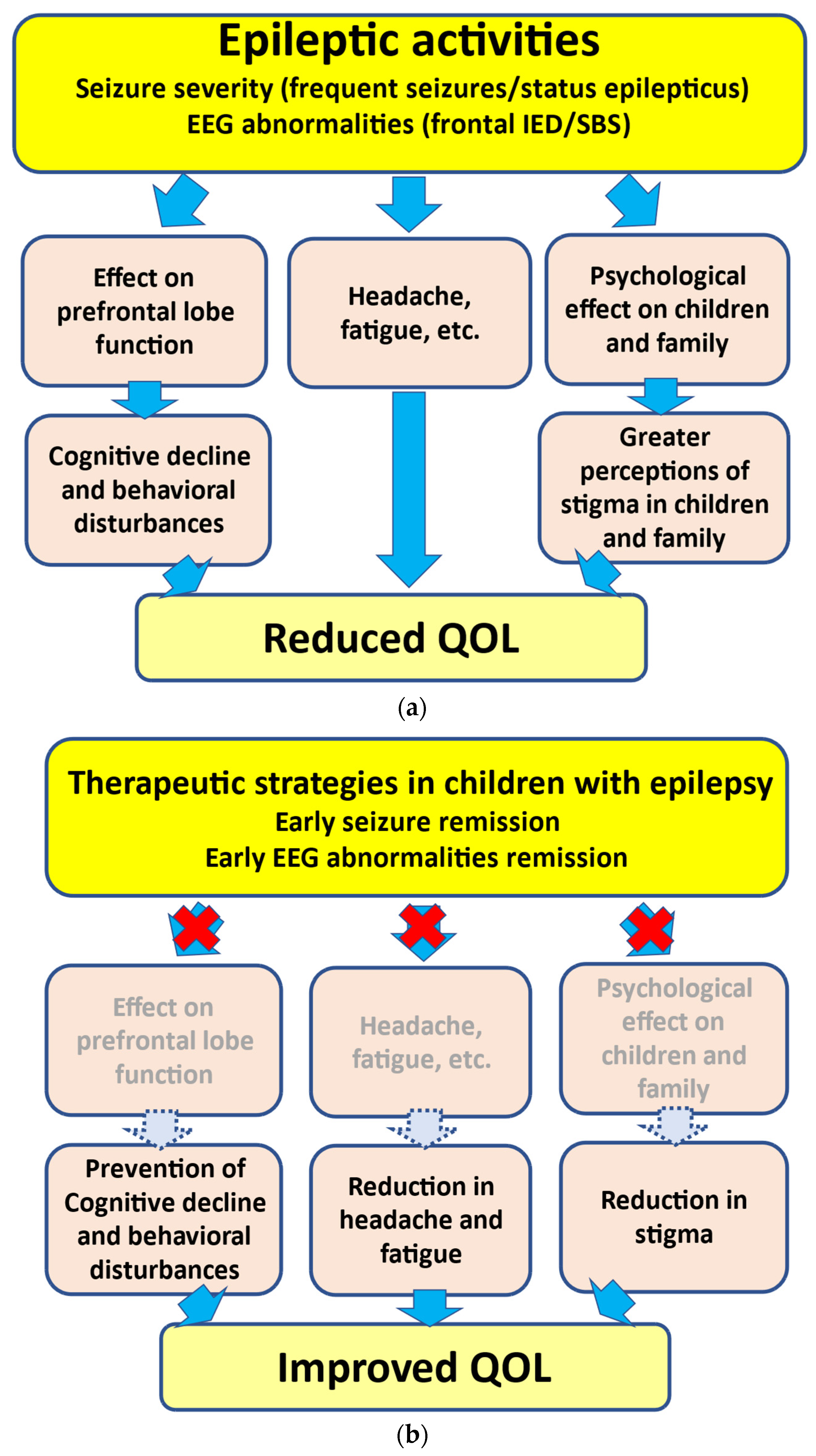
3.2. Is the Urgent Suppression of IEDs on EEG Needed?
As inferred from various studies, frequent IEDs and frontal IEDs can lead to neuropsychological impairments [41][35] (Figure 1). Reductions in IEDs on EEG may be related to behavioral improvements in ADHD/ASD children with frontal IEDs with or without clinical seizures [44,46,53][36][37][38]. Accordingly, frontal IEDs can lead to neurodevelopmental deterioration in ADHD/ASD, and ASM treatment may be effective for both IED reduction and behavioral improvement in children with ADHD/ASD with frontal IEDs. With respect to EECSWS-related neurodevelopmental deterioration, previous studies have underlined the parallel course of EECSWS and neuropsychological impairments [54][39]. Neuropsychological impairments may appear concurrently with EEG abnormalities [55][40] (Figure 1). Moreover, these impairments may improve concurrently with the disappearance of EEG abnormalities rather than clinical seizures. In children with SBS, behavioral improvements can be associated with EEG improvement [48][41]. These findings suggest that the active phase of “epilepsy”, not only “clinical seizures”, can be a prognostic factor, and the urgent suppression of IEDs, such as SBS, may thus be warranted to prevent neurodevelopmental deterioration in children presenting with SBS [56][42].3.3. Therapeutic Strategies in Children with Epilepsy
Based on these findings, the urgent suppression of clinical seizures and EEG abnormalities, such as frontal IEDs and SBS, may be required to prevent neuropsychological impairments. During ASM selection and adjustment, physicians should strategize the therapeutic approach to controlling seizures and suppressing EEG abnormalities in children with epilepsy. Among the various ASMs, novel ASMs, such as levetiracetam and perampanel, may suppress both clinical seizures and IEDs on EEG [48,57,58,59][41][43][44][45]. These novel ASMs may represent an important addition to the treatments available for epileptic children presenting with frontal IEDs and SBS. However, it remains unclear whether seizure presence or remission are in any way related to environmental factors.References
- Terra, V.C.; Furlanetti, L.L.; Nunes, A.A.; Thome, U.; Nishiyama, M.A.; Sakamoto, A.C.; Machado, H.R. Vagus nerve stimulation in pediatric patients: Is it really worthwhile? Epilepsy Behav. 2014, 31, 329–333.
- Chung, S.; Szaflarski, J.P.; Choi, E.J.; Wilson, J.C.; Kharawara, S.; Kauer, G.; Hirsch, L.J. A systematic review of seizure clusters: Prevalence, risk factors, burden of disease and treatment patterns. Epilepsy Res. 2021, 177, 106748.
- Kanemura, H.; Sano, F.; Tando, T.; Sugita, K.; Aihara, M. Repeated seizures induce prefrontal growth disturbance in frontal lobe epilepsy. Brain Dev. 2012, 34, 175–180.
- Kanemura, H.; Sano, F.; Ohyama, T.; Aoyagi, K.; Sugita, K.; Aihara, M. Sequential prefrontal lobe volume changes and cognitive dysfunctions in children with Panayiotopoulos syndrome presenting with status epilepticus. Epilepsy Res. 2015, 112, 122–129.
- Binnie, C.D. Cognitive impairment during epileptiform discharges: Is it ever justifiable to treat the EEG? Lancet Neurol. 2003, 2, 725–730.
- Aydemir, N.; Ozkara, C.; Unsal, P.; Canbeyli, R. A comparative study of health related quality of life, psychological well-being, impact of illness and stigma in epilepsy and migraine. Seizure 2011, 20, 679–685.
- Espinola-Nadurille, M.; Crail-Melendez, D.; Sanchez-Guzman, M.A. Stigma experience of people with epilepsy in Mexico and views of health care providers. Epilepsy Behav. 2014, 32, 162–169.
- Hamelin, S.; Kahane, P.; Vercueil, L. Fatigue in epilepsy: A prospective inter-ictal and post-ictal survey. Epilepsy Res. 2010, 91, 153–160.
- Hernandez-Ronquillo, L.; Moien-Afshari, F.; Knox, K.; Britz, J.; TellezZenteno, J.F. How to measure fatigue in epilepsy? The validation of three scales for clinical use. Epilepsy Res. 2011, 95, 119–129.
- Weglage, J.; Demsky, A.; Pietsch, M.; Kurlemann, G. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev. Med. Child Neurol. 1997, 39, 646–651.
- Hoare, P. The development of psychiatric disorder among school children with epilepsy. Dev. Med. Child Neurol. 1984, 26, 3–13.
- Austin, J.K.; Risinger, M.W.; Beckett, L.A. Correlates of behavior problems in children with epilepsy. Epilepsia 1992, 33, 1115–1122.
- Holmes, G.L.; Ben-Ari, Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr. Res. 2001, 49, 320–325.
- Kanemura, H.; Hata, S.; Aoyagi, K.; Sugita, K.; Aihara, M. Serial changes of prefrontal lobe growth in the patients with benign childhood epilepsy with centrotemporal spikes presenting with cognitive impairments/behavioral problems. Brain Dev. 2011, 33, 106–113.
- Sinclair, D.B.; Wheatley, M.; Snyder, T. Frontal lobe epilepsy in childhood. Pediatr. Neurol. 2004, 30, 169–176.
- Williamson, P.D.; Spencer, D.D.; Spencer, S.S.; Novelly, R.A.; Mattson, R.H. Complex partial seizures of frontal lobe origin. Ann. Neurol. 1985, 18, 497–504.
- Engel, J., Jr.; Williamson, P.D.; Wieser, H.G. Mesial temporal lobe epilepsy with hippocampal sclerosis. In Epilepsy. A Comprehensive Textbook, 2nd ed.; Engel, J., Pedley, T., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 2479–2486.
- Fejerman, N.; Caraballo, R.H. Early-onset benign childhood occipital epilepsy (Panayiotopoulos type). In Benign Focal Epilepsies in Infancy, Childhood and Adolescence; Fejerman, N., Caraballo, R.H., Eds.; John Libbey Eurotext: Montrouge, France, 2007; pp. 115–144.
- Andermann, F. Clinical features of migraine–epilepsy syndrome. In Migraine and Epilepsy; Andermann, F., Lugaresi, E., Eds.; Butterworths: Boston, MA, USA, 1987; pp. 3–30.
- Kanemura, H.; Sano, F.; Ishii, S.; Ohyama, T.; Sugita, K.; Aihara, M. Characteristics of headache in children with epilepsy. Seizure 2013, 22, 647–650.
- Vercoulen, J.H.; Hommes, O.R.; Swanink, C.M.; Jongen, P.J.; Fennis, J.F.; Galama, J.M.; van der Meer, J.W.M.; Bleijenberg, G. The measurement of fatigue in patients with multiple sclerosis. A multidimensional comparison with chronic fatigue syndrome and healthy subjects. Arch. Neurol. 1996, 53, 642–649.
- Dittner, A.J.; Wessely, S.C.; Brown, R.G. The assessment of fatigue: A practical guide for clinicians and researchers. J. Psychosom. Res. 2004, 56, 157–170.
- Christensen, D.; Johnsen, S.P.; Watt, T.; Harder, I.; Kirkevold, M.; Andersen, G. Dimensions of post-stroke fatigue: A two-year follow-up study. Cerebrovasc. Dis. 2008, 26, 134–141.
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Association between seizure frequency and fatigue levels in children with epilepsy. J. Paediatr. Child Health 2018, 54, 1336–1340.
- Jacoby, A.; Baker, G.; Smith, D.; Dewey, M.; Chadwick, D. Measuring the impact of epilepsy: The development of a novel scale. Epilepsy Res. 1993, 16, 83–88.
- Rodenburg, R.; Meijer, A.M.; Dekovic, M.; Aldenkamp, A.P. Parents of children with enduring epilepsy: Predictions of parenting stress and parenting. Epilepsy Behav. 2007, 11, 197–207.
- Modi, A.C. The impact of a new pediatric epilepsy diagnosis on parents: Parenting stress and activity patterns. Epilepsy Behav. 2008, 13, 169–173.
- Chiou, H.H.; Hsieh, L.P. Parenting stress in patients of children with epilepsy and asthma. J. Child Neurol. 2008, 23, 301–306.
- Cushner-Weinstein, S.; Dassoulas, K.; Salpekar, J.A.; Henderson, S.E.; Pearl, P.L.; Gaillard, W.D.; Weinstein, S.L. Parenting stress and childhood epilepsy: The impact of depression, learning, and seizure-related factors. Epilepsy Behav. 2008, 13, 109–114.
- Kerne, V.; Chapieski, L. Adaptive functioning in pediatric epilepsy: Contributions of seizure-related variables and parental anxiety. Epilepsy Behav. 2015, 43, 48–52.
- Braams, O.; Meekes, J.; Braun, K.; Schappin, R.; van Rijen, P.C.; Hendriks, M.P.H.; Jennekens-Schinkel, A.; van Nieuwenhuizen, O. Parenting stress does not normalize after child’s epilepsy surgery. Epilepsy Behav. 2015, 42, 147–152.
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Seizure severity in children with epilepsy is associated with their parents’ perception of stigma. Epilepsy Behav. 2016, 63, 42–45.
- Austin, J.K.; Dunn, D.W.; Caffrey, H.M.; Perkins, S.M.; Harezlak, J.; Rose, D.F. Recurrent seizures and behavior problems in children with first recognized seizures: A prospective study. Epilepsia 2002, 43, 1564–1573.
- Aihara, M.; Aoyagi, K.; Goldberg, E.; Nakazawa, S. Age shifts frontal cortical control in a cognitive bias task from right to left: Part I. Neuropsychological study. Brain Dev. 2003, 25, 555–559.
- Kanemura, H.; Sano, F.; Aoyagi, K.; Sugita, K.; Aihara, M. Do sequential EEG changes predict atypical clinical features in rolandic epilepsy? Dev. Med. Child Neurol. 2012, 54, 912–917.
- Kanemura, H.; Sano, F.; Tando, T.; Hosaka, H.; Sugita, K.; Aihara, M. EEG improvements with antiepileptic drug treatment can show a high correlation with behavior recovery in children with ADHD. Epilepsy Behav. 2013, 27, 443–448.
- Kanemura, H.; Sano, F.; Hoshino, H.; Aihara, M. Efficacy of perampanel in epilepsy patients with autism spectrum disorder. Epilepsy Res. 2021, 170, 106550.
- Kanemura, H.; Sano, F.; Ohyama, T.; Sugita, K.; Aihara, M. Effect of levetiracetam on behavioral problems in pervasive developmental disorder children with epilepsy. Eur. J. Paediatr. Neurol. 2014, 18, 482–488.
- De Negri, M.; Baglietto, M.G.; Battaglia, F.M.; Gaggero, R.; Pessagno, A.; Recanati, L. Treatment of electrical status epilepticus by short diazepam (DZP) cycles after DZP rectal bolus test. Brain Dev. 1995, 17, 330–333.
- Morikawa, T.; Seino, M.; Yagi, K. Long-term outcome of four children with continuous spike-waves during sleep. In Epileptic Syndromes in Infancy, Childhood and Adolescence, 2nd ed.; Roger, J., Bureau, M., Dravet, C.H., Dreifuss, F.E., Perret, A., Wolf, P., Eds.; John Libbey: London, UK, 1992; pp. 257–266.
- Kanemura, H.; Sano, F.; Hoshino, H.; Takayama, K.; Aihara, M. Effects of perampanel on secondary bilateral synchrony and behavioral problems in adolescents with epilepsy showing insufficient response with levetiracetam. Seizure 2020, 80, 131–137.
- Kanemura, H.; Aihara, M. Neurobiological effects of CSWS on brain growth: A magnetic resonance imaging volumetric study. J. Pediatr. Epilepsy 2012, 1, 187–193.
- Stodieck, S.; Steinhoff, B.J.; Kolmsee, S.; Van Rijckevorsel, K. Effect of levetiracetam in patients with epilepsy and interictal epileptiform discharges. Seizure 2001, 10, 583–587.
- Kanemura, H.; Sano, F.; Ohyama, T.; Aihara, M. Efficacy of levetiracetam for reducing rolandic discharges in comparison with carbamazepine and valproate sodium in rolandic epilepsy. Seizure 2018, 62, 79–83.
- Wu, T.; Ido, K.; Ohgoh, M.; Hanada, T. Mode of seizure inhibition by sodium channel blockers, an SV2A ligand, and an AMPA receptor antagonist in a rat amygdala kindling model. Epilepsy Res. 2019, 154, 42–49.
