Term infants with specific pathologies and preterm infants are frequently unable to feed and need parenteral nutrition (PN) to provide adequate calories and necessary nutrients that promote growth and sustain essential biological functions. Amino acid (AA) solutions are an integral part of standard PN administered in neonatal intensive care units (NICUs). These AA solutions contain variable amounts of both essential and nonessential AAs. A low plasma level of any AA indicates a relative deficiency of this AA, which may be detrimental to nitrogen balance, growth and the specific functions related to this AA. Multiple studies confirmed low plasma cysteine in parenterally fed neonates. Cysteine plays an important role in glutathione (GSH) synthesis, which is a cornerstone in keeping the oxidant–antioxidant balance in preterm infants who are exposed to high oxidative stress induced mainly by O2 supplementation and PN contaminated with peroxide. To respond to the urgent need for changing the current methods of parenteral cysteine supplementation, glutathione addition to PN is presented as an innovative alternative with promising results in an animal model. F At the end of this review, future directions for research in this field are proposed.
1. Cysteine Sources
Cysteine is available through gut absorption from the diet
[1][14]. In the case of infants on
parenteral nutrition (PN
), some
amino acid (AA)AA solutions contain cysteine, while others provide only methionine as a pro-cysteine AA. The different AA solutions and their content of cysteine and methionine are discussed
in Section 4 of this review. The endogenous synthesis of cysteine with the sulfur atom of methionine and the carbon skeleton of serine makes it a dispensable amino acid under normal conditions
[2][15]. As depicted in
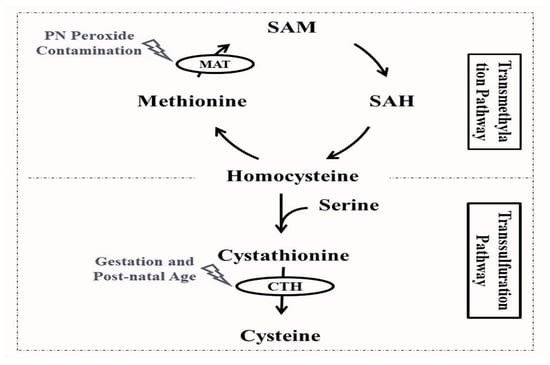
Figure 1, the first steps of cysteine synthesis are closely linked to the methionine cycle: methionine is converted by the action of the methionine adenosyltransferase into S-adenosylmethionine, which is subsequently converted to S-adenosylhomocysteine, and homocysteine. The following steps of cysteine synthesis take part in the transsulfuration pathway, where homocysteine is coupled to serine by the action of cystathionine-β-synthase, forming cystathionine, which will ultimately lead to cysteine through the cleavage action of γ-cystathionase
[3][16].
Figure 1. Cysteine endogenous synthesis through the transmethylation and transsulfuration pathways and affected enzyme activities among preterm infants. CTH: γ-cystathionase enzyme; MAT: methionine adenosyltransferase enzyme; PN: parenteral nutrition; SAH: S-adenosylhomocysteine; SAM: S-adenosylmethionine.
2. Is Cysteine a Conditionally Essential Amino Acid in Preterm Infants?
For the past decades, it has been unclear if cysteine is an essential amino acid for preterm infants
[4][35]. To better understand the situations that can make cysteine a conditionally essential AA, this section describes the factors affecting cysteine bioavailability.
2.1. Factors Affecting Plasma Cysteine Concentration Subsection
Besides the nutritional intake of cysteine, there are several factors that can affect plasma cysteine. Clinically, these factors are associated with gestational age, postnatal age and the use of PN. The biological mechanisms can be resumed in the following two important steps of cysteine synthesis.
2.1.1. Maturity of the Transsulfuration Pathway
The final step of the transsulfuration pathway leading to cysteine synthesis is catalyzed by the cleaving action of γ-cystathionase (
Figure 1)
[3][16]. Whereas some studies reported that the activity of this key enzyme is undetectable in the liver of fetuses or preterm infants that died during the first 24 h of life
[5][6][36,37], others demonstrated that hepatic cystathionase activity in the first two weeks of life is reduced by half in preterm in comparison to full-term infants and appears to be positively correlated with gestational and postnatal age
[7][38]. Similar findings were reported in animal studies; the hepatic cystathionase activity was lower in fetal and neonatal rats compared to adult rats
[8][39]. The measurable activity of hepatic cystathionase in preterm infants
[7][38] and the evident rates of transsulfuration among full-term enterally fed and preterm infants parenterally fed
[9][40] may demonstrate the preterm capacity to synthesize cysteine but do not ensure that de novo cysteine synthesis is sufficient, particularly under major oxidative stress
[10][41].
This lower hepatic cystathionase activity is associated with higher plasma cystathionine and lower cysteine concentrations in extremely and very preterm infants in comparison to moderate/late preterm and term infants
[11][32]. The de novo synthesis of cysteine is dependent on the maturity of the transsulfuration pathway that is tightly linked to gestational age and postnatal age. Studies demonstrated that plasma cysteine concentrations on the first day of life are positively correlated with gestational age
[12][42] or birth weight
[13][34]. Whereas some authors reported no postnatal changes in plasma cysteine concentrations up to two weeks of life of preterm infants receiving PN
[14][15][43,44], others reported a significant increase in plasma and erythrocyte cysteine concentration on day 7 of life of extremely and very low birth weight infants receiving parenteral and some enteral nutrition
[12][42].
2.1.2. Activity of the Transmethylation Pathway
The first step of the transmethylation pathway and endogenous cysteine synthesis is catalyzed by the action of methionine adenosyltransferase, converting methionine into S-adenosylmethionine (
Figure 1)
[3][16]. This enzyme is a sensitive target of free radicals, and its activity is affected by oxygen, nitrogen radical species
[16][45] and peroxides
[17][46]. The gut immaturity of preterm infants requires the use of PN to ensure growth and survival
[4][35]. This essential nutrition is, however, inherently contaminated with peroxides (due to the nutrients’ interaction with dissolved oxygen), causing a redox imbalance and deleterious consequences of oxidative stress in preterm infants
[18][47]. PN peroxide contamination was shown to reduce methionine adenosyltransferase activity in newborn guinea pigs, leading to the more oxidized redox potential of glutathione
[17][46].
2.2. Factors Affecting Intracellular Cysteine Availability
Besides the plasma cysteine concentration, another important factor that limits the intracellular cysteine availability is the maturity of the cellular cysteine uptake. Evidence of the sex and gestational age-dependent maturity of the cysteine uptake of cells in preterm infants suggests that even with a sufficient plasma cysteine concentration, some extremely preterm infants could have depleted intracellular cysteine
[19][22].
In the light of these factors, it is expected that preterm infants less than 32 weeks of gestation on PN have the highest risk of cysteine depletion. This mechanistic comprehension can help with understanding the results of the randomized controlled trials conducted by Riedjik et al. concluding that, in very low birth weight infants with a gestational age < 29 weeks (
n = 47) at one month of postnatal age (at 32 ± 0 and 36 ± 1 weeks post-menstrual age) tolerating full enteral feeds
[20][48] and moderate preterm infants (gestational age 32–34 weeks) fully enterally fed (
n = 25)
[21][49] randomized to five different formulas only differing by cysteine concentrations, cysteine is not considered a conditionally essential amino acid. In these two studies, infants were ≥32 weeks PMA post menstrual age and fully enterally fed. Their cellular cysteine uptake, as well as the transsulfuration pathway, would be more mature. Furthermore, the oxidation-sensitive transmethylation pathway would not be affected as these infants were not receiving any PN. In these two studies, formula enrichment in cysteine ensured an exogenous supply of this amino acid
[20][21][48,49]. Using the same mechanistic comprehension approach can help with understanding the results confirming that cysteine is a conditionally essential amino acid in preterm infants < 32 weeks due to decreased γ-cystathionase activity
[11][32].
3. A New Promising Alternative for Parenteral Cysteine Supplementation
Due to its abundance in cells and its high cysteine content, which represents a third of its composition, glutathione (γ-glutamylcysteinylglycine) is considered as a cysteine reservoir for the organism
[22][23][30,72]. Due to its γ-glutamyl group, glutathione cannot freely cross the cell membrane. Cells must therefore synthesize their own glutathione. In the 1970s and 1980s, the golden age of enzymology, Alton Meister pioneered the understanding of glutathione metabolism. Among other things, he proposed the ‘γ-glutamyl cycle’
[24][25][73,74]. Since then, the metabolism that supports this cycle has been used and cited frequently by scientists. Recently, in 2018, the metabolism of this cycle was revisited by Bachhawat AK and Yadav S.
[26][75], who confirmed much of it while clarifying certain notions in the light of more recent knowledge. A summary of
our
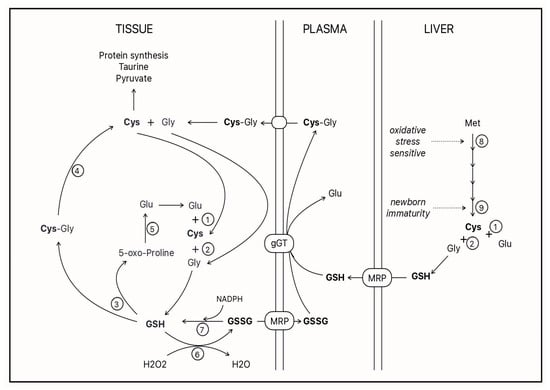
esearchers' current understanding of cysteine-glutathione metabolism is depicted in (
Figure 2).
Figure 2. GSH: glutathione; GSSG: disulfide glutathione; Cys: cysteine; Gly: glycine; Glu: glutamate; Met: methionine; MRP: multidrug resistance protein; gGT: gamma-glutamyl transpeptidase. Open circles represent enzymes 1: gamma-glutamyl ligase; 2: GSH synthetase; 3: gamma-glutamyl cyclotransferase; 4: dipeptidases; 5: 5-oxo-prolinase; 6: glutathione peroxidase; 7: glutathione reductase; 8: methionine adenosyltransferase; and 9: cystathionase.
The ectoenzyme γ-glutamyl transpeptidase (γ GT) is key in this cycle
[27][28][76,77]. It hydrolyzes the gamma–peptide bond of glutathione, releasing the dipeptide cysteinylglycine, which is captured by the cell, where dipeptidases release the cysteine into the cytosol
[29][78]. The natural substrates of γ GT are molecules containing a γ-glutamyl group, such as reduced glutathione (GSH), disulfide glutathione (GSSG) and glutathione-conjugated molecules
[26][30][31][75,79,80]. The affinity of the enzyme for these forms of glutathione varies between 6 and 12 µM
[30][79], whereas the normal concentration of glutathione in plasma is reported to be between 3 and 12 µM
[22][23][32][29,30,72]. Unfortunately, the plasma concentration of glutathione in one-week-old premature infants is 1.2 ± 0.1 μM
[33][12]. This low plasma glutathione can explain the low glutathione level measured in leukocytes of premature infants
[34][81]. A cysteine deficiency in these infants has been demonstrated
[35][36][37][2,3,4].
Knowing that γ GT, which matures in the first days of life of premature neonates
[38][33], has a similar affinity for GSH and GSSG,
our
esearchers' question was which form of glutathione is most suitable to act as a supplement to PN. Of course, GSH reactivity predicts interactions with other nutrients present in intravenous solutions. The better stability of GSSG, predicted by its chemical structure, was confirmed. After 24 h of incubation of the PN at room temperature, 11% glutathione remained in the GSH-supplemented PN solutions compared to 72% in the GSSG-supplemented solutions
[39][82]. The 28% loss of glutathione in the GSSG-supplemented solution was explained by the disulfide exchange between the GSSG and cysteine present in the PN, generating the mixed disulfide cysteine-glutathione
[40][83]. This latter molecule is also a substrate for γ GT providing cysteine to cells for de novo glutathione synthesis
[41][84].
The GSSG supplementation of PN made it possible to prevent, in newborn guinea pigs, a deficiency in plasma and pulmonary glutathione
[42][43][85,86]. By preventing pulmonary oxidative stress, this supplementation has also prevented the pulmonary loss of alveoli
[6][38][33,37], which is a main characteristic of bronchopulmonary dysplasia observed in premature infants.