Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Viviana De Caro and Version 2 by Rita Xu.
Oral Mucositis, a debilitating side effect of radio and chemotherapy for head and neck cancers, involves inflammation and ulceration of the mucous membranes in the oral cavity. This condition often leads to severe pain, difficulty in eating, and compromised quality of life for cancer patients. The use of natural compounds such as polyphenols has shown promise in preventing and alleviating Oral Mucositis as they possess anti-inflammatory, antioxidant, and healing properties, capable of mitigating the adverse effects of chemo and radiotherapy on the oral mucosa.
- oral mucositis
- polyphenols
- head and neck cancers
- phytocomplex
- curcuminoids
1. Introduction
Head and neck cancers are common tumours, representing approximately 10% of all malignancies in men and 5% in women [1]. They originate in the upper aero-digestive tract at various levels including the larynx, upper trachea, pharynx, and oral and nasal cavity [2]. The onset of these diseases is currently upward and seems to be related to some diffuse risk factors such as consumption of alcohol and tobacco (separately or in combination) and virus infections (e.g., Human Papilloma Virus—HPV) [1][3][1,3]. Among the head and neck cancers, oral cancers are remarkably relevant for human health and about 90% of them belong to the squamous cell carcinoma type. In particular, Oral Squamous Cell Carcinoma (OSCC) represents the most frequent malignant tumour affecting the oral mucosal epithelium, with a higher incidence in the male population [4], although a share of OSCC appears to involve an increasingly younger population (under 40). Despite the progress in therapy, the mortality of patients with OSCC has remained steadily high during the last 20 years as compared to other cancers [5]. Its early detection and treatment are still crucial to improve the prognosis, and, in this regard, a combination of multiple therapeutic approaches is frequently recommended to remove cancer and prevent any recurrence [6]. Conventional therapies include surgery, radiotherapy, and chemotherapy; however, these are characterized by several side effects and low patient compliance. Therefore, other more specific treatments such as localized and personalized therapy (which target some genes in cancer cells), administration of natural molecules as adjuvant and chemopreventive agents [7][8][7,8], as well as immunotherapy with monoclonal antibodies could be employed [9]. The routine recommendation is surgical management of the primary tumour followed by post-operative radiotherapy or chemoradiotherapy depending on the presence of intermediate/high risk. However, radio- and chemotherapy currently remain the most common therapeutic strategies [10]. Chemotherapy is generally based on drugs such as 5-Fluorouracil (5-FU), Cisplatin, Cetuximab, and Taxanes which being non-selective drugs [11][12][11,12] are characterized by several side effects such as gastrointestinal disorders, immune and hematopoietic deficit with consequent infections, inflammation and mucositis in the entire gastrointestinal tract including the oral cavity. On the other hand, head and neck radiotherapy shows unpleased local effects such as severe hyposalivation/xerostomia due to impairment of normal salivary gland function, damage of the oral mucosa, and dermatitis in the overlying epithelium [13]. Major damage to the oral cavity occurs if chemotherapy is associated with radiotherapy as their synergistic effect in the reduction of the epithelial cell turnover leads to thinning of the mucosa itself causing a loss of integrity. Therefore, a compromised function of the epithelium together with a strong inflammation process, oxidative environment, and high susceptibility to bacterial infections often originate in a complex clinical scenario defined as Oral Mucositis (OM) [14]. Considering that oral cancer itself is a disabling disease with a low survival rate, the onset of OM during treatments could further compromise the patient’s overall conditions, lowering their quality of life due to the severe repercussions on nutrition and other mouth functions (as speaking, digesting, or even simply opening the mouth).
2. Oral Mucositis (OM)
OM is a severe acute inflammation affecting the oral mucosa characterized by tissue swelling, ulceration, and erythema [15]. OM develops in around 40–60% of patients with head and neck cancer who receive standard radiotherapy and chemoradiotherapy [16]. OM is described as a painful condition generally resulting in hard discomfort and a negative impact in terms of patients’ quality of life. Depending on the cancer treatment used, it is characterized by a different onset. The common signs that characterize OM are erythema, erosions, and ulcerations of the tissues, which cause a painful condition that can be severe, depending on the size and localization of the damage to the mucous membrane [17][18][17,18]. This condition can be further worsened by bacterial infections [4]. In this regard, the World Health Organization (WHO) developed a grading system for the OM severity, ranging from 0 (no OM) to 4 (highly severe OM), which is used to assess its clinical features such as symptoms (e.g., pain), signs (e.g., erythema and ulceration), and oral function (e.g., swallowing ability). This scale also evaluates the severity of clinical manifestations which depends on several factors, such as the type of treatment, the dose, and such individual variables (response to treatment, age, diet, oral hygiene, tumour type, and genetic factors) [19]. Although the onset of OM is the result of different mechanisms that may occur simultaneously, it is possible to identify a common sequence of events that characterizes the pathogenesis of both chemo- and radiotherapy-induced OM. The model described by Sonis in 2004 [20] is useful for the recognition of this sequence of five events:
-
Inflammatory/vascular phase: It is induced by chemotherapy and/or radiotherapy, which induce cytotoxicity in normal cells by directly damaging the DNA and leading to excessive ROS generation. This phenomenon acts as a trigger for the inflammatory process activating different signalling pathways such as proinflammatory cytokines (e.g., IL1 β, IL6, and TNF-α) and prostaglandins [21][22][21,22].
-
Activation of transcription factors such as nuclear factor-κ B (NF-κB) and NF-E2-related factor 2 (Nrf2) which can be directly activated by the chemotherapeutic agents, e.g., 5-FU activates the NF-κB, thereby upregulating the genes encoding pro-inflammatory cytokines such as Tumour Necrosis Factor α (TNF-α), interleukin 1β (IL-1β), and IL-6, cyclooxygenase 2 (COX-2), and high-mobility group box 1 protein (HMGB1) or radiation, and indirectly through the ROS release, producing inflammatory mediators, which increase the tissue damage stimulating angiogenesis and vascular permeability [23].
-
Up-regulation and signal amplification stage, leading to loss of the epithelium integrity and, hence, ulcer formation (beginning of OM evolution).
-
Rich inflammatory infiltrate stage, containing macrophages, neutrophils, and mastocytes [24]. In addition, lesions are strongly subjected to bacterial colonization, which contributes to stimulating the innate immune system, thereby increasing the inflammatory response.
-
The final healing phase, characterized by the proliferating and differentiating epithelial cells, leading to the restoration of the integrity of altered mucosa.
Almost 20–40% of cancer patients undergoing conventional chemotherapy manifest this condition (CT-OM) within 4–7 days of treatment, and the clinical signs and symptoms continue for 1 to 2 weeks after.
In head and neck cancer patients, immunotherapy is amongst the most promising strategies. Moreover, the prevalence of related OM seems to be lower compared to traditional chemotherapeutics (methotrexate, cisplatin) [25].
However, OM represents a potential side effect of both targeted therapy and immunotherapy (mammalian target of rapamycin inhibitor-associated stomatitis (mIAS) and immunotherapy-related adverse events (irAEs)) with both severity and clinical presentation depending on the agent used [26]. When combined with conventional chemotherapy, these therapies may increase the risk and severity of mucosal involvement, with a combined presentation of both superficial and deeper, classic Oral Mucositis ulcers [27].
Moreover, Amy et al. reported severe chronic OM associated with pembrolizumab immunotherapy lasting for months even after the drug was stopped and representing the major cause of suffering and eating difficulties for cancer patients [28].
Also, radiation therapy for head and neck cancer causes OM; in this case, side effect onset is delayed, but the duration is longer (between 2 and 6 weeks). Radiation therapy-induced OM (RT-OM) depends on the dose administered, the volume of the tissue treated, and the type of radiation used. The sequelae of CT/RT-OM, including pain, odyno/dysphagia, dysgeusia, decreased oral intake, and local/systemic infection, often require treatment delays, interruptions, and discontinuations which have negative impacts not only on the quality of life but also on tumour control and survival [29].
Currently, the clinical management of OM is mainly aimed at alleviating symptoms, and it is therefore based on the use of conventional drugs suitable for treating pain, inflammation, and infection such as topical anaesthetics (e.g., lidocaine), systemic opioid analgesics (e.g., morphine and fentanyl), and topical and systemic antibiotics and antifungals, which are generally recommended for the prevention of infection [30][31][30,31]. Treatment of oral mucosal irAEs is generally carried out with high-potency topical steroids, or systemic steroid/immunosuppressive agents [26].
However, preventing and/or treating any recurrence remains quite complex and often requires multiple approaches which can cause multiple side effects. Therefore, the identification of alternative ways to prevent and treat CT and RT–OM is necessary, especially considering the new targets recently discovered in the pathogenesis of mucosal damage [32].
As the abnormal release of Reactive Oxygen Species (ROS) and the massive inflammatory phase are the main causes of the onset and recurrence of OM, the use of natural antioxidants and anti-inflammatory compounds could be a wise strategy. Among these, polyphenols have recently emerged [33].
Polyphenols in the Prevention and Management of OM
Naturally, plants produce a wide variety of molecules in response to various environmental stimuli (e.g., microbial infection, temperature, etc.). The same compounds, due to their natural actions, could also show interesting properties in humans, useful in the prevention and treatment of several disorders [34][35][36][34,35,36]. Among the wide variety of plant-derived bioactive molecules, polyphenols represent a class of secondary metabolites that are widely distributed in the plant kingdom and are generally synthesized as a defense against such environmental stresses and pathogens [37]. Tea, cocoa powder, grapes, and spices are just a few examples of rich natural sources of polyphenols, but they are also found in edible plants and natural products such as propolis and honey [38].
The large class of polyphenols comprise a broad group of phytochemicals that includes hundreds of natural molecules which are divided into different subclasses according to their main chemical structure. These compounds range from small molecules such as phenolic acids or stilbenes to larger molecules such as tannins [39]. The chemical characteristic that units all of the polyphenols are a phenolic structure based on phenyl ring(s) as a core which presents one or more hydroxyl substituents, essential for the radical scavenging activity (due to electron delocalization and stabilization of the formed radical) [37]. According to their structure polyphenols can be classified into two main groups: flavonoids and non-flavonoids (Figure 1). Flavonoids possess two phenyl rings fused with a central heterocyclic ring containing oxygen [40]. They can be further subdivided into flavonols, flavones, flavanones, flavanols isoflavones, anthocyanidins, and chalcones.
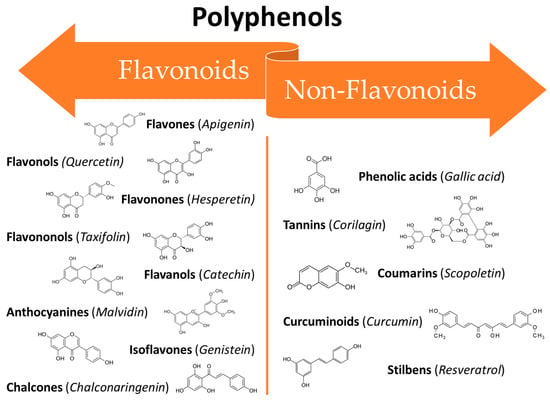
Figure 1. Classification of polyphenols: main classes and subclasses with some representative molecules.
On the other hand, the non-flavonoids can be divided into different subclasses such as phenolic acids, tannins, coumarins, curcuminoids, and stilbenes [41]. In recent years, polyphenols have gained the attention of researchers all around the world thanks to their broad spectrum of bioactive properties, which may be useful in improving human health, as well as their low side effects. They can interact with different cellular pathways, enzymatic mechanisms, and hormones controlling gene expression [42]. Thus, the mechanisms involved in their activity are complex and often still controversial. Their wide range of biological activities includes antioxidant [38], antifungal [43][44][43,44], anti-inflammatory [45], anti-aging [46], osteogenic [47], chemopreventive [48], and antitumoural [49] properties which makes them potentially useful in several fields ranging from pharmaceuticals to cosmetics [50]. Several in vitro studies, in vivo studies, and clinical trials have been conducted to assess the efficacy of polyphenols against the oral mucosa damage that occurs as a side effect of anticancer therapies. The broad-spectrum activities of polyphenols make them ideal candidates for clinical use in many areas of medicine. However, their use is still limited due to their unfavourable physicochemical properties such as low water solubility, instability at high temperatures and alkaline pH, as well as a massive first-pass effect after oral administration, resulting in extremely low bioavailability [51]. Recently, researchers have proposed several innovative solutions to mitigate these drawbacks in order to benefit from the therapeutic effects of these potent molecules. Some of the most recent innovations are related to new techniques for their extraction and manipulation and the design of innovative drug delivery platforms and systems suitable for minimizing the degradation phenomena and improving bioavailability [51].
3. Curcuminoids
Curcuminoids are the main active components of the rhizomes of Curcuma longa L., an herb widely used as a traditional remedy in China and Southeast Asia. The term “curcuminoids” indicates a mixture of compounds such as curcumin, Dimethoxy curcumin, and Bis-dimethoxy curcumin. Furthermore, there is also a fourth molecule called Cyclocurcumin, which was initially identified as curcuminoid but was later considered only to be a structural isomer of curcumin [52]. Curcuminoids are composed of a core structure having two aromatic benzene methoxy rings linked by an unsaturated seven-carbon chain consisting of an α,β-unsaturated β-diketone. The presence of this specific group ensures the pH-dependent keto-enol tautomerism [53]. This keto-enol balance is very important for the physico-chemical and antioxidant properties of curcuminoids. Considering curcumin as an example, when it is in the enolic form both aromatic rings can interact, delocalizing the electrons present in the π orbitals. The chemical structure of curcuminoids also permits a wide range of beneficial and therapeutic effects as antimicrobial [54], anti-inflammatory [55][56][55,56], neuroprotective [57], and anticancer [58]. Curcumin (CUR, Figure 2), the most studied of the curcuminoids, exerts its anti-inflammatory effects through upregulation of the peroxisome proliferator-activated receptor gamma (PPAR-γ) [59] and downregulation of NF-Kβ, thereby suppressing the subsequent synthesis of cytokines such as TNF-α, IL-1β, IL-6 and IL-8 and vascular endothelial growth factor (VEGF) [60]. It increases the plasma levels of superoxide dismutase (SOD) and glutathione peroxidase, enhancing the catalase activity and reducing the plasma levels of lipid peroxidase. CUR is able to participate in many signalling pathways by modulating several signalling molecules (e.g., pro-apoptotic proteins, COX-2, C-reactive protein, prostaglandin E2) and adhesion molecules [61].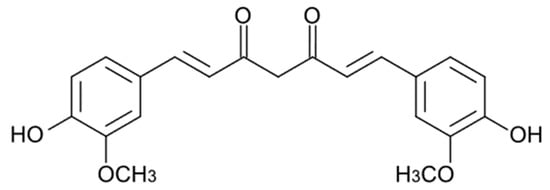
Figure 2. Chemical structure of curcumin.
