Numerous surgeries are carried out to replace tissues that have been harmed by an illness or an accident. Due to various surgical interventions and the requirement of bone substitutes, the emerging field of bone tissue engineering attempts to repair damaged tissues with the help of scaffolds. These scaffolds act as template for bone regeneration by controlling the development of new cells. For the creation of functional tissues and organs, there are three elements of bone tissue engineering that play very crucial role: cells, signals and scaffolds. For the achievement of these aims, various types of natural polymers, like chitosan, chitin, cellulose, albumin and silk fibroin, have been used for the preparation of scaffolds. Scaffolds produced from natural polymers have many advantages: they are less immunogenic as well as being biodegradable, biocompatible, non-toxic and cost effective. The hierarchal structure of bone, from microscale to nanoscale, is mostly made up of organic and inorganic components like nanohydroxyapatite and collagen components. This review paper summarizes the knowledge and updates the information about the use of natural polymers for the preparation of scaffolds, with their application in recent research trends and development in the area of bone tissue engineering (BTE).
- tissue engineering
- nano-scaffolds
- biopolymers
- nanostructure
- bone regeneration
1. Natural Polymers
2. Chitosan and Chitin
2.1. Applications of Chitin/Chitosan in Bone Tissue Engineering
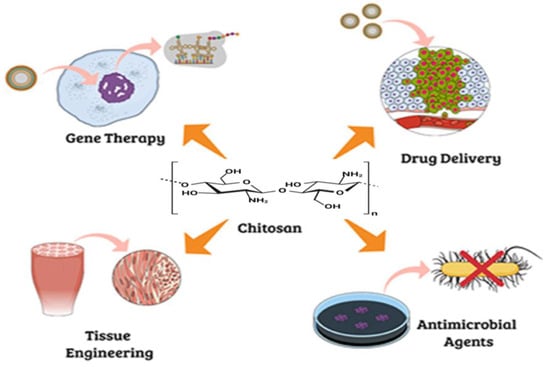
1.2. Application of Chitin/Chitosan in Bone Implants and Healing of Osteoarthritis Defects
1.3. Application of Chitosan/Chitin in Periodontal Regeneration
2. Cellulose
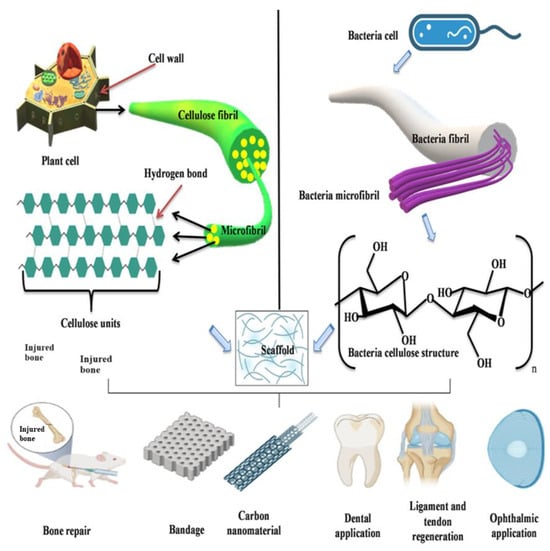
2.1. Application of Plant Cellulose in Bone Tissue Engineering
2.2. Applications of Bacterial Cellulose for Bone Regeneration
3. Albumin
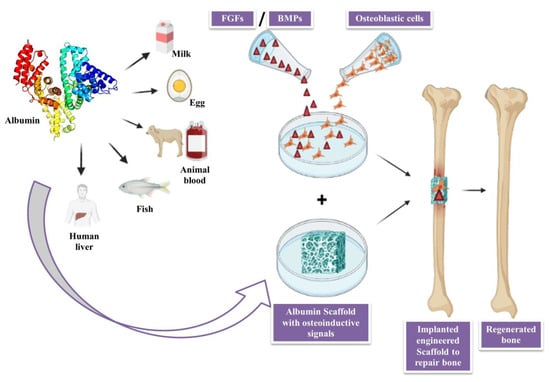
3.1. Applications of Albumin in Bone Tissue Engineering
3.2. Role of Albumin as Biomaterial in Regenerative Medicine
3.3. Role of Albumin as a Nano-Scaffolds
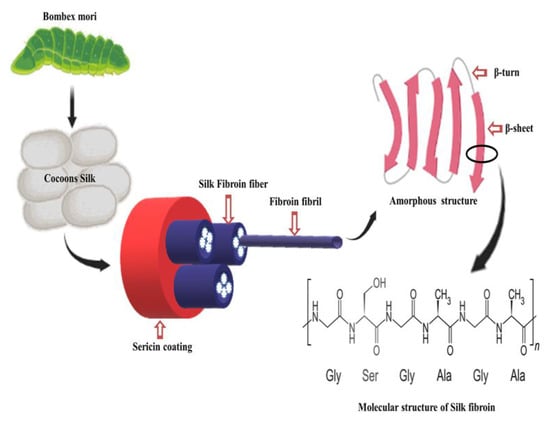
4. Silk Fibroin
References
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. Biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–37.
- Nagarajan, S.; Radhakrishnan, S.; Kalkura, S.N.; Balme, S.; Miele, P.; Bechelany, M. Overview of protein-based biopolymers for biomedical application. Macromol. Chem. Phys. 2019, 220, 1900126.
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105.
- Sivakumar, P.M.; Yetisgin, A.A.; Sahin, S.B.; Demir, E.; Cetinel, S. Bone tissue engineering: Anionic polysaccharides as promising scaffolds. Carbohydr. Polym. 2022, 283, 119142.
- Iber, B.T.; Kasan, N.A.; Torsabo, D.; Omuwa, J.W. A review of various sources of chitin and chitosan in nature. J. Renew. Mater. 2022, 10, 1097.
- Dey, S.C.; Al-Amin, M.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. pH induced fabrication of kaolinite-chitosan biocomposite. Int. Lett. Chem. Phys. Astron 2016, 68, 1–9.
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430.
- Vander Straeten, A.; Lefèvre, D.; Demoustier-Champagne, S.; Dupont-Gillain, C. Protein-based polyelectrolyte multilayers. Adv. Colloid Interface Sci. 2020, 280, 102161.
- Jaworska, M.M.; Antos, D.; Górak, A. Review on the application of chitin and chitosan in chromatography. React. Funct. Polym. 2020, 152, 104606.
- Craciun, A.-M.; Mititelu-Tartau, L.; Gavril, G.; Marin, L. Chitosan crosslinking with pyridoxal 5-phosphate vitamer toward biocompatible hydrogels for in vivo applications. Int. J. Biol. Macromol. 2021, 193, 1734–1743.
- Kaviani, A.; Zebarjad, S.M.; Javadpour, S.; Ayatollahi, M.; Bazargan-Lari, R. Fabrication and characterization of low-cost freeze-gelated chitosan/collagen/hydroxyapatite hydrogel nanocomposite scaffold. Int. J. Polym. Anal. Charact. 2019, 24, 191–203.
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402.
- Saravanan, S.; Leena, R.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365.
- Karbasi, S.; Alizadeh, Z.M. Effects of multi-wall carbon nanotubes on structural and mechanical properties of poly (3-hydroxybutyrate)/chitosan electrospun scaffolds for cartilage tissue engineering. Bull. Mater. Sci. 2017, 40, 1247–1253.
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739.
- Islam, S.; Bhuiyan, M.R.; Islam, M. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866.
- Moreira, C.D.; Carvalho, S.M.; Sousa, R.G.; Mansur, H.S.; Pereira, M.M. Nanostructured chitosan/gelatin/bioactive glass in situ forming hydrogel composites as a potential injectable matrix for bone tissue engineering. Mater. Chem. Phys. 2018, 218, 304–316.
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 1–14.
- Cortellini, P.; Tonetti, M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontology 2000 2015, 68, 282–307.
- Petit, C.; Batool, F.; Bugueno, I.M.; Schwinté, P.; Benkirane-Jessel, N.; Huck, O. Contribution of statins towards periodontal treatment: A review. Mediat. Inflamm. 2019, 2019, 6367402.
- Sah, A.K.; Dewangan, M.; Suresh, P.K. Potential of chitosan-based carrier for periodontal drug delivery. Colloids Surf. B Biointerfaces 2019, 178, 185–198.
- Kamal, T.; Khan, S.B.; Haider, S.; Alghamdi, Y.G.; Asiri, A.M. Thin layer chitosan-coated cellulose filter paper as substrate for immobilization of catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2017, 104, 56–62.
- Ganguly, A.; Ian, C.K.; Sheshala, R.; Sahu, P.S.; Al-Waeli, H.; Meka, V.S. Application of diverse natural polymers in the design of oral gels for the treatment of periodontal diseases. J. Mater. Sci. Mater. Med. 2017, 28, 39.
- Barreras, U.S.; Méndez, F.T.; Martínez, R.E.M.; Valencia, C.S.; Rodríguez, P.R.M.; Rodríguez, J.P.L. Chitosan nanoparticles enhance the antibacterial activity of chlorhexidine in collagen membranes used for periapical guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 1182–1187.
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial cellulose: A smart biomaterial with diverse applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623.
- Courtenay, J.C.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Bae, Y.; Scott, J.L.; Sharma, R.I. Modulating cell response on cellulose surfaces; tunable attachment and scaffold mechanics. Cellulose 2018, 25, 925–940.
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.; Ragauskas, A.J. Recent advances in functional materials through cellulose nanofiber templating. Adv. Mater. 2021, 33, 2005538.
- Mohajer, S.; Rezaei, M.; Hosseini, S.F. Physico-chemical and microstructural properties of fish gelatin/agar bio-based blend films. Carbohydr. Polym. 2017, 157, 784–793.
- Aghazadeh, M.R.; Delfanian, S.; Aghakhani, P.; Homaeigohar, S.; Alipour, A.; Shahsavarani, H. Recent advances in development of natural cellulosic non-woven scaffolds for tissue engineering. Polymers 2022, 14, 1531.
- Allen, H.; Wei, D.; Gu, Y.; Li, S. A historical perspective on the regulation of cellulose biosynthesis. Carbohydr. Polym. 2021, 252, 117022.
- Zhong, C. Industrial-scale production and applications of bacterial cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374.
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial cellulose and its applications. Polymers 2022, 14, 1080.
- Cakmak, A.M.; Unal, S.; Sahin, A.; Oktar, F.N.; Sengor, M.; Ekren, N.; Gunduz, O.; Kalaskar, D.M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymers 2020, 12, 1962.
- Oprea, M.; Pandele, A.M.; Nicoara, A.I.; Nicolescu, A.; Deleanu, C.; Voicu, S.I. Crown ether-functionalized cellulose acetate membranes with potential applications in osseointegration. Int. J. Biol. Macromol. 2023, 230, 123162.
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Miculescu, F. Characterization and in vitro and in vivo assessment of a novel cellulose acetate-coated Mg-based alloy for orthopedic applications. Materials 2017, 10, 686.
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V.K. Sericin covalent immobilization onto cellulose acetate membrane for biomedical applications. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774.
- Corobea, M.S.; Albu, M.G.; Ion, R.; Cimpean, A.; Miculescu, F.; Antoniac, I.V.; Raditoiu, V.; Sirbu, I.; Stoenescu, M.; Voicu, S.I. Modification of titanium surface with collagen and doxycycline as a new approach in dental implants. J. Adhes. Sci. Technol. 2015, 29, 2537–2550.
- Pandele, A.; Comanici, F.; Carp, C.; Miculescu, F.; Voicu, S.; Thakur, V.; Serban, B. Synthesis and characterization of cellulose acetate-hydroxyapatite micro and nano composites membranes for water purification and biomedical applications. Vacuum 2017, 146, 599–605.
- Saska, S.; Barud, H.; Gaspar, A.; Marchetto, R.; Ribeiro, S.J.L.; Messaddeq, Y. Bacterial cellulose-hydroxyapatite nanocomposites for bone regeneration. Int. J. Biomater. 2011, 2011, 175362.
- Lee, Y.-J.; An, S.-J.; Bae, E.-B.; Gwon, H.-J.; Park, J.-S.; Jeong, S.I.; Jeon, Y.-C.; Lee, S.-H.; Lim, Y.-M.; Huh, J.-B. The effect of thickness of resorbable bacterial cellulose membrane on guided bone regeneration. Materials 2017, 10, 320.
- Lee, S.-H.; Lim, Y.-M.; Jeong, S.I.; An, S.-J.; Kang, S.-S.; Jeong, C.-M.; Huh, J.-B. The effect of bacterial cellulose membrane compared with collagen membrane on guided bone regeneration. J. Adv. Prosthodont. 2015, 7, 484–495.
- Amorim, W.L.; Costa, H.O.; Souza, F.C.d.; Castro, M.G.d.; Silva, L.d. Experimental study of the tissue reaction caused by the presence of cellulose produced by Acetobacter xylinum in the nasal dorsum of rabbits. Rev. Bras. De Otorrinolaringol. 2009, 75, 200–207.
- Yuan, H.; Van Blitterswijk, C.; De Groot, K.; de Bruijn, J.D. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 78, 139–147.
- Zhang, H.; Wang, J.; Wang, K.; Xu, L. A bilayered PLGA/multiwall carbon nanotubes/bacterial cellulose composite membrane for tissue regeneration of maxillary canine periodontal bone defects. Mater. Lett. 2018, 212, 118–121.
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623.
- Bolaños, K.; Kogan, M.J.; Araya, E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int. J. Nanomed. 2019, ume 14, 6387–6406.
- Jiang, X.; Mu, H.; Hsieh, Y.-H.P.; Rao, Q. Isolation and Characterization of Chicken Serum Albumin (Hen Egg Alpha-Livetin, Gal d 5). Foods 2022, 11, 1637.
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum albumin: A multifaced enzyme. Int. J. Mol. Sci. 2021, 22, 10086.
- Horvathy, D.B.; Vacz, G.; Szabo, T.; Szigyarto, I.C.; Toro, I.; Vamos, B.; Hornyak, I.; Renner, K.; Klara, T.; Szabo, B.T. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 126–132.
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 10557.
- Aiyelabegan, H.T.; Zaidi, S.S.; Fanuel, S.; Eatemadi, A.; Ebadi, M.T.; Sadroddiny, E. Albumin-based biomaterial for lung tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 853–861.
- Nosrati, H.; Salehiabar, M.; Manjili, H.K.; Danafar, H.; Davaran, S. Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. Int. J. Biol. Macromol. 2018, 108, 909–915.
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B Biointerfaces 2020, 192, 111041.
- Prasopdee, T.; Sinthuvanich, C.; Chollakup, R.; Uttayarat, P.; Smitthipong, W. The albumin/starch scaffold and its biocompatibility with living cells. Mater. Today Commun. 2021, 27, 102164.
- Lv, L.; Chi, Y.; Chen, C.; Xu, W. Structural and functional properties of ovalbumin glycated by dry-heating in the presence of maltodextrin. Int. J. Food Prop. 2015, 18, 1326–1333.
- Swiercz, R.; Mo, M.; Khare, P.; Schneider, Z.; Ober, R.J.; Ward, E.S. Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget 2017, 8, 3528.
- Li, P.-S.; -Liang Lee, I.; Yu, W.-L.; Sun, J.-S.; Jane, W.-N.; Shen, H.-H. A novel albumin-based tissue scaffold for autogenic tissue engineering applications. Sci. Rep. 2014, 4, 5600.
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol. Pharm. 2021, 18, 1862–1894.
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159.
- Visentini, F.F.; Perez, A.A.; Santiago, L.G. Bioactive compounds: Application of albumin nanocarriers as delivery systems. Crit. Rev. Food Sci. Nutr. 2023, 63, 7238–7268.
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867.
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of oriented collagen fiber scaffolds and its application in bone tissue engineering. Appl. Mater. Today 2021, 22, 100902.
- Kong, F.; Mehwish, N.; Lee, B.H. Emerging albumin hydrogels as personalized biomaterials. Acta Biomater. 2023, 157, 67–90.
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide–Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032.
- Vasu, S.; Zhou, J.; Chen, J.; Johnston, P.V.; Kim, D.-H. Biomaterials-based approaches for cardiac regeneration. Korean Circ. J. 2021, 51, 943–960.
- Raut, H.K.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of biomaterials for tissue regeneration or replacement. Biotechnol. J. 2020, 15, 2000160.
- Punj, S.; Singh, J.; Singh, K. Ceramic biomaterials: Properties, state of the art and future prospectives. Ceram. Int. 2021, 47, 28059–28074.
- Horváthy, D.B.; Schandl, K.; Schwarz, C.M.; Renner, K.; Hornyák, I.; Szabó, B.T.; Niculescu-Morzsa, E.; Nehrer, S.; Dobó-Nagy, C.; Doros, A. Serum albumin-coated bone allograft (BoneAlbumin) results in faster bone formation and mechanically stronger bone in aging rats. J. Tissue Eng. Regen. Med. 2019, 13, 416–422.
- An, F.-F.; Zhang, X.-H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667.
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056.
- Fu, Q.-W.; Zi, Y.-P.; Xu, W.; Zhou, R.; Cai, Z.-Y.; Zheng, W.-J.; Chen, F.; Qian, Q.-R. Electrospinning of calcium phosphate-poly (D, L-lactic acid) nanofibers for sustained release of water-soluble drug and fast mineralization. Int. J. Nanomed. 2016, ume 11, 5087–5097.
- Patel, D.; Haag, S.L.; Patel, J.S.; Ytreberg, F.M.; Bernards, M.T. Paired Simulations and Experimental Investigations into the Calcium-Dependent Conformation of Albumin. J. Chem. Inf. Model. 2022, 62, 1282–1293.
- Zhang, C.; Zhang, Y.; Shao, H.; Hu, X. Hybrid silk fibers dry-spun from regenerated silk fibroin/graphene oxide aqueous solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358.
- Gore, P.M.; Naebe, M.; Wang, X.; Kandasubramanian, B. Progress in silk materials for integrated water treatments: Fabrication, modification and applications. Chem. Eng. J. 2019, 374, 437–470.
- von Byern, J.; Chandler, P.; Merritt, D.; Adlassnig, W.; Stringer, I.; Meyer-Rochow, V.B.; Kovalev, A.; Dorrer, V.; Dimartino, S.; Marchetti-Deschmann, M. Biomechanical properties of fishing lines of the glowworm Arachnocampa luminosa (Diptera; Keroplatidae). Sci. Rep. 2019, 9, 3082.
- Klein, B.A. Wax, wings, and swarms: Insects and their products as art media. Annu. Rev. Entomol. 2022, 67, 281–303.
- Narimani, M.; Teimouri, A.; Shahbazarab, Z. Synthesis, characterization and biocompatible properties of novel silk fibroin/graphene oxide nanocomposite scaffolds for bone tissue engineering application. Polym. Bull. 2019, 76, 725–745.
- Fang, G.; Sapru, S.; Behera, S.; Yao, J.; Shao, Z.; Kundu, S.C.; Chen, X. Exploration of the tight structural–mechanical relationship in mulberry and non-mulberry silkworm silks. J. Mater. Chem. B 2016, 4, 4337–4347.
- Malay, A.D.; Sato, R.; Yazawa, K.; Watanabe, H.; Ifuku, N.; Masunaga, H.; Hikima, T.; Guan, J.; Mandal, B.B.; Damrongsakkul, S. Relationships between physical properties and sequence in silkworm silks. Sci. Rep. 2016, 6, 27573.
- Dou, H.; Zuo, B. Effect of sodium carbonate concentrations on the degumming and regeneration process of silk fibroin. J. Text. Inst. 2015, 106, 311–319.
- Reizabal, A.; Costa, C.M.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Silk fibroin as sustainable advanced material: Material properties and characteristics, processing, and applications. Adv. Funct. Mater. 2023, 33, 2210764.
- Qiu, W.; Liu, X.Y. Recent progress of applying mesoscopic functionalization engineering principles to spin advanced regenerated silk fibroin fibers. Adv. Fiber Mater. 2022, 4, 390–403.
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499.
