Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Klaudia Marek and Version 4 by Mona Zou.
There is a wide variety of tools and measures for rehabilitation outcomes in post-stroke patients with impairments in the upper limb and hand, such as paralysis, paresis, flaccidity, and spasticity. However, there is a lack of general recommendations for selecting the most appropriate scales, tests, and instruments to objectively evaluate therapy outcomes. Reviews on upper limb and hand measurements reveal that clinicians’ choices of tools and methods are highly varied. Some clinicians and medical teams continue to employ non-standard and unverified metrics in their research and measurements.
- outcome measures
- post-stroke rehabilitation
- upper limb
- hand measurements
- stroke recovery
1. Introduction
There is no consensus regarding the outcome measures necessary to assess upper limb function in post-stroke patients [1]. In the acute and subacute phases, patients within six months after a stroke undergo a comprehensive and specialized evaluation of their health [2]. This process, held in hospital clinics, involves determining the rehabilitation and treatment goals, selecting therapeutic interventions, and evaluating progress [3]. The successful rehabilitation of post-stroke patients relies on effective motor deficit assessments, accurate diagnoses and therapy choices, proper outcome evaluations, and prolonged treatment and rehabilitation to maintain recovery in the chronic phase [4]. There are multiple perspectives from which hand outcome measures can be examined, highlighting the complexity of assessing rehabilitation progress. These perspectives include physiological measures that focus on the physical changes and improvements, quality of life measures that assess the impact on the patients’ daily lives and well-being, phase of stroke recovery to determine the appropriate stage-specific interventions, and the type of hospital care the patient requires, which may vary based on individual needs and progress. The motor impairment of the upper limb after a stroke contributes to weakened muscle strength. Low muscle strength is associated with fatigue phenomena, reduced endurance, and ineffective task performance by the patient [5]. These are the main problems that occur in hemiparesis [6]. The image of a patient with hemiparesis after stroke is heterogeneous [7]. Static and isokinetic dynamometers are used to obtain objective results of muscle endurance as well as muscle strength itself [5]. Patients in the chronic phase with paresis receive reeducation of the number of motor units and long-term denervation of paresis muscles, which contributes to muscle weakness [8]. Manual dexterity can be a valuable predictor of motor impairment after stroke [9]. The impairment of the above function leads to reduced manual dexterity, limiting activities of daily living and worsening quality of life. After a stroke, it turns out that there are difficulties with basic activities, such as cooking, laundry, cleaning, and many others [10]. In particular, two-handed activities that require a high degree of manual dexterity are difficult to perform [11]. Hand grip strength (HSG) is another parameter that checks the return of muscle strength. It is a useful measurement and a prognostic biomarker after stroke. It is performed using the Jamar dynamometer along with a standardized test protocol approved by the American Society of Hand Therapists (ASHT) [12][13]. Impaired motor function after stroke often occurs with muscle spasticity, resulting in poorer motor recovery of the affected limb. In this case, the main problems are increased muscle tension, which requires checking the resistance of the muscles to stretching but also the range of motion of the joints (ROM) and pain due to deformities and contractures [14][15]. The failure of the joints to move individually is among the features of hemiparesis of the upper limb. There is difficulty in controlled movement of the limb during reaching, which is caused by abnormal torque production and impaired interjoint coordination [16][17]. Understanding and considering these different perspectives can help healthcare professionals develop a more comprehensive and tailored approach to hand rehabilitation for stroke patients. In physiotherapy diagnostics, instruments are used in addition to tests and scales. Centimeter tapes and a goniometer designed for measurement are widely available and simple in service [18]. The need to obtain accurate results led to the development of many tools used in the diagnosis of stroke patients, including hand dynamometers, haptic sensors, position tracking systems, leap motion, and the use of artificial intelligence. Hand measurement is essential in the diagnostic process in the context of enabling individualization of therapy [19][20][21][22].
To maintain a stroke patient’s health, it is crucial to start rehabilitation as soon as possible and, once the inpatient rehabilitation process is complete, to continuously monitor the patient’s condition and rehabilitate after leaving the hospital [23]. The intensity of rehabilitation is correlated with better inpatient rehabilitation outcomes. Higher doses of therapy (increased intensity) during inpatient rehabilitation have a positive impact on the functional independence measure (FIM), leading to an increase in its levels [24]. Despite widespread access to hand and arm measurement scales and tests, there is no general agreement on which measures are best for evaluating therapy for post-stroke patients in the chronic phase. Bushnell et al. recommend using the Fugl-Meyer Upper Extremity scale, the Wolf Motor Function Test, and the Action Research Arm Test for upper limb and hand measurements [25]. They emphasize that the Fugl-Meyer Upper Extremity scales should be the primary outcome measure in the chronic phase of stroke. In the acute and subacute phases of stroke, there are no clear procedures regarding which measures should be performed to assess outcomes. This lack of clarity leads to discrepancies between evidence and practice in making hand diagnoses [26]. Furthermore, Murphy et al., 2021 endorsed the use of the Fugl-Meyer Upper Extremity scale and the Action Research Arm Test measures for patients [26].
Upper limb limitations, motor deficits, and hemiplegia are present in more than 80% of post-stroke patients [27]. These impairments impact coordination and manual dexterity, which play a crucial role in daily life and functioning [28]. About 67% of individuals who have experienced a stroke with complications still cannot use their hand, even four years after the event [29]. Upper limb spasticity, which can develop following a stroke, affects 17–40% of people [30][31]. During the restoration of motor function after a stroke, cortical reorganization and synaptic plasticity mechanisms occur in the brain. Both cerebral hemispheres are involved in these reorganization processes [32]. Their activity is highest within the first few months following the disease [27][32]. The unique critical period, also referred to as the sensitive period, comprises the first three months after a stroke, during which complete recovery is possible [33].
Reduced hand mobility is linked to difficulties in performing daily tasks. Challenges with grasping objects, transferring, manipulating, coordinating hand-finger movements, and maintaining dexterity are both rehabilitation obstacles and targets [34][35]. Even up to 12 months post-stroke, daily use of the affected upper limb is three times less frequent compared to the healthy limb [36].
There is a need to understand the assessment of the physical and physiological conditions and associated measurements of a stroke patient’s hand. There are different degrees of spontaneous improvement in arm paresis within the first months after stroke. An assessment of the patient’s improvement in physical condition and hand function after 6 months can be predicted based on the results of motor deficits occurring after 1 month of hospitalization, despite a further 5 months of routine rehabilitation [30]. The hand’s biomechanical complexity is reflected in the large portion of the brain’s motor cortex dedicated to controlling hand movements. The precision of hand movement control is highly dependent on the intact corticospinal tract [37][38][39]. Hand rehabilitation is based on motor movement, which plays an important role in the brain’s motor cortex [40]. The corticospinal tract (the compensatory corticoreticulospinal tract branches) branches in many segments in the spinal cord and innervates more proximal muscles than distal ones, with a predominance of flexors, but it lacks resolution and also innervates the extensors of the fingers and hands. This causes abnormal involuntary coupling between shoulder visitation and wrist/finger flexion (flexion synergy), as well as weakness in the muscles that are the extensors of the distal joints. This results in a significant limitation of hand opening movement [41][42].
The ability to actively extend the fingers up to 7 days after a stroke is part of the prognosis for recovery of upper limb mobility. Independent extension of the wrist and each finger is also key, which is associated with the integrity of the corticospinal tract. Also, a prognostic factor is the movement of visiting the absence within 72 h, thumb extension, and the movement of closing and opening the hand [43][44][45][46][47]. The latter movement represents multi-finger movement, which is important because of the frequent impairment of multi-finger coordination in post-stroke patients [48]. Carpinella et al. showed that inadequate finger extension is due to two concurrent causes: altered neurophysiological control mechanisms and mechanical limitation of the extension movement [49]. Mechanical limitations can be caused by atrophy of the extensor muscles, contracture of the flexor muscles, increased passive stiffness of the muscle tissue, or the shortening of muscle fibers. Neurophysiological disorders can result from flexor muscle spasticity, a weakness of the extensor muscles, or excessive contraction of the extensor and flexor muscles [49][50][51][52][53][54]. Robotic rehabilitation can lead to the alleviation of the occurrence of spasticity in the wrist joint [55]. Restoration of normal muscle tone is a predictive factor, marking the first stage of recovery from the onset of the disease [56]. Researchers indicate that the Brunnstrom Stages of Stroke Recovery are useful in assessing motor recovery in post-stroke patients.
The growing discussion about the selection and correct execution of measurements assessing patients’ functional status and objective outcomes accelerates the development of precision medicine in the field of rehabilitation. Alfano et al. explain precision medicine using oncology treatment and rehabilitation as an example by linking the right treatment to the patient, determining the exact analysis and location of the disease, and patient characteristics. Precision medicine can positively influence rehabilitation, reducing patients’ overall suffering caused by the disease and helping to maintain their quality of life, such as functioning, ability to work, and active participation in society [57]. However, precision medicine is not as widely developed in the field of rehabilitation. In the future, identifying and combining biomarkers that develop in stroke may answer questions about the selection of appropriate treatment and the risk of complications of cerebrovascular disease [58], which will, in turn, impact functional assessment and rehabilitation therapy tailored to the patient’s capabilities.
A major goal of rehabilitation is to improve the quality of life of post-stroke patients. The quality of life is related to the perception of the disease’s impact on physical activity, emotional activity, and society functioning [59][60]. Proper observation of recovery is carried out in accordance with the International Classification of Functioning, Disability, and Health (ICF) [61]. The ICF distinguishes two main frameworks: Functioning and Disability, which includes the categories of Body Structures and Activity and Participation, and a second contextual factor containing environmental and personal factors [62]. The goal of the framework used in the ICF is to standardize the description of health made by clinicians in rehabilitation [63]. Researchers who systematize and review rehabilitation outcome measures point out that there is a lack of instruments to assess actual outcomes. They indicate that new outcome assessment tools useful in different patient populations need to be developed [64]. Psychometric properties, including reliability, validity, and responsiveness, are a vital concept in selecting appropriate and effective outcome measures and instruments [65]. According to van Gils (2018), the assessment of the upper limb of a stroke patient should consider motor impairment, activity, and ambidextrous performance simultaneously [66]. The Fugl-Meyer test for studying post-stroke patients is the common choice among researchers. It is a good measure to track changes in returning function in the upper limb [67].
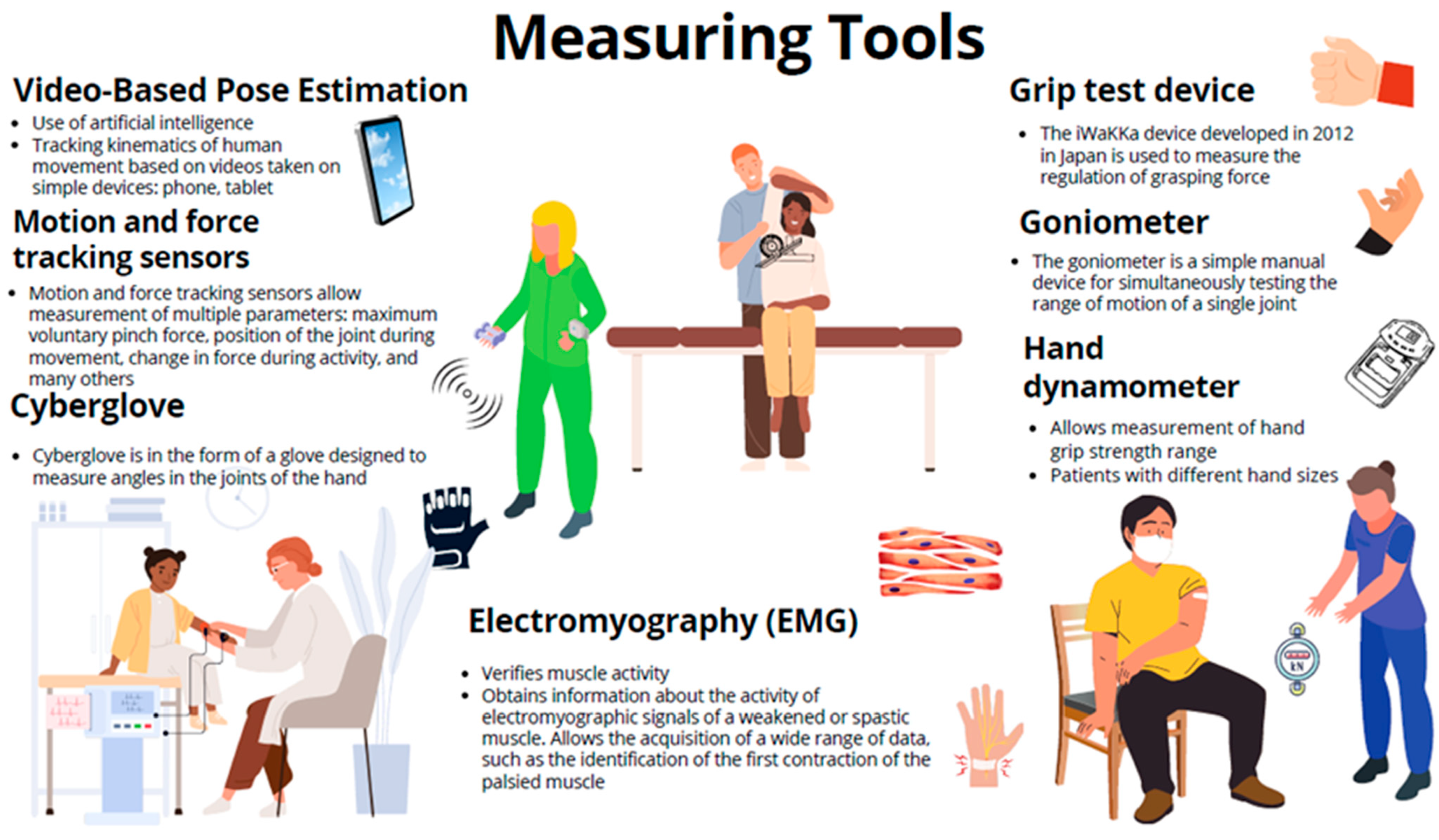
2. Diagnosis, Hand Measurements, and Instruments Support: Results
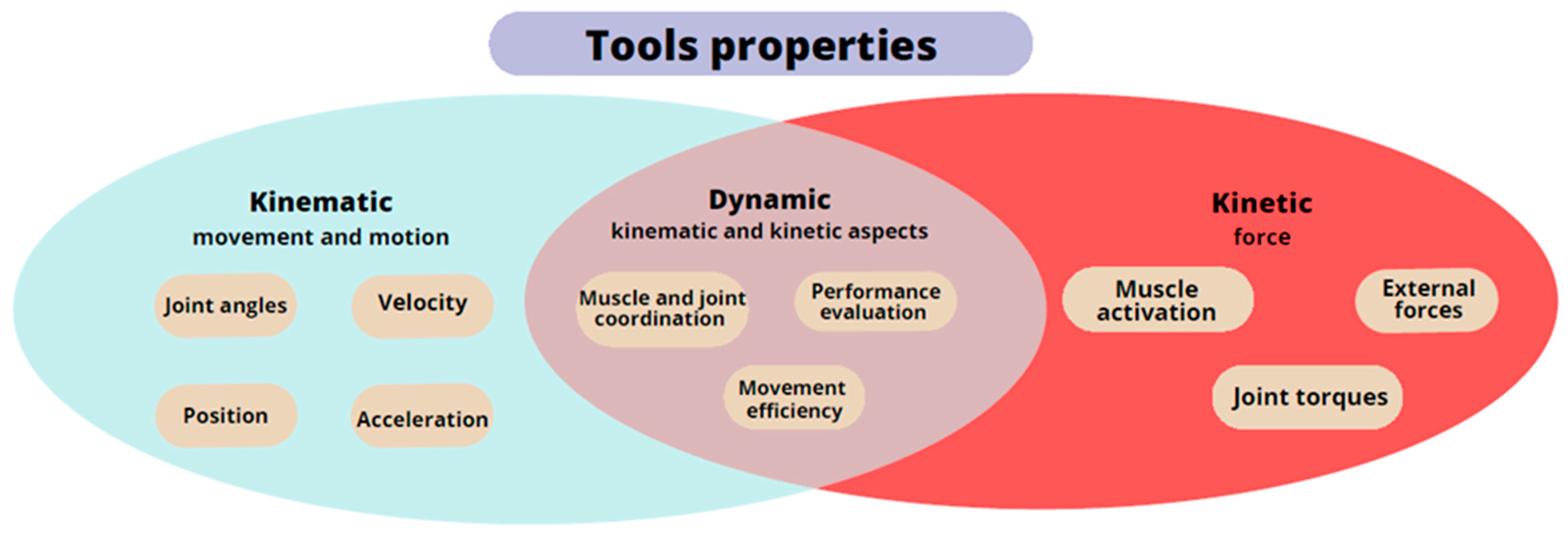
The selection of scales for assessing post-stroke motor impairment is extensive, and they encompass various movements, including synergistic movements, manual skills, grasping, manipulation of objects of different sizes, range of motion in joints, etc. (Figure 2) [68]. Some of these measures have limited sensitivity or exhibit a ceiling effect, as they are unable to capture the entire scope of impairment. Additionally, measurements taken by different medical personnel can lead to varying results and reduced objectivity [4][69][70][71]. The literature on stroke rehabilitation describes numerous instruments and scales for evaluating patients and their recovery [69]. An analysis of the instruments used in stroke research reveals significant heterogeneity in the choice of assessment measure and method of use [72]. It is not uncommon to encounter studies employing non-standard and unvalidated measures [73]. Stroke patients experience various stages of upper limb and hand complications, as described by Brunnstrom’s stages of recovery. The Brunnstrom Stages of Stroke Recovery, described in 1966, is still one of the most frequently used standards for clinical assessment after stroke. It should be remembered that the scale is subjective in character, and the assessment depends heavily on the experience of the clinician. Lack of experience may be reflected in inconsistencies in assessment results based on the scale [74]. Initially, the patient’s hand exhibits reduced muscle tone, known as flaccid paralysis, which is followed by spasticity until selective control of movement develops. When muscle tension normalizes, normal movement is restored [75]. The time it takes to transition from the flaccid phase to the phase of increased muscle tone varies but typically ranges from one to three weeks after stroke onset [76]. For some patients, flaccid paralysis may persist for years and is defined as prolonged muscle hypotonia lasting more than two months after a stroke [77]. Persistent flaccid paralysis for over a year following the stroke predicts poorer and slower rehabilitation outcomes for the affected hand [77][78]. Flaccid paralysis is associated with lower neuron and peripheral nervous system syndrome and, in addition to decreased muscle tone, it can be characterized by muscle atrophy, weakness, and absence of reflexes [79][80]. Spasticity is a phenomenon in which the integration of the motor response of the nervous system to sensory stimuli is impaired. Typically defined as a velocity-dependent increase in muscle tension, spasticity is associated with hypersensitivity of the reflex arc. It is a component of upper motor neuron syndrome, the symptoms of which can include hypertonia, contractures, and movement disorders. Spasticity refers to changes occurring in the central nervous system [81][82]. To bolster the reliability of measurement results, clinicians are progressively turning to specialized instruments (Figure 1 and Figure 2). These instruments are categorized based on the specific properties they measure: kinematic, kinetic, and dynamic. The kinematic properties focusing on the motion and movement can be measured using goniometers, motion capture systems, and inertial measurement units. Kinetic properties pertaining to forces that act on the hand and contribute to the movement can be assessed using force sensors, dynamometers (grip test devices and hand dynamometers), and myographic devices (electromyography and mechanomyography). Dynamic properties encompassing both aspects and the hand function during activities can be measured using instrumented gloves (CyberGlove), robotic devices, and 3D scanner-based integrated vision systems, such as Microsoft Kinect or Leap Motion [21].
Figure 1. Measuring tools used as outcome measures in post-stroke hand rehabilitation.

Figure 2. Diagram of the properties of the hand measurement tools.
3. Discussion
In the future, the number of post-stroke individuals requiring rehabilitation is expected to increase. Estimations suggest that by 2030, the global count of post-stroke patients will reach 70 million [83]. Many of these individuals will experience complications related to their hands and upper limbs, making it crucial to develop effective therapies that enable active participation in daily life without limitations [84]. Recovery and proper movement after a stroke are possible due to cerebral plasticity. Learning motor skills can lead to lasting changes in motor behavior, which is desirable for rehabilitation [85]. A major global priority is identifying effective interventions and rehabilitation therapies for stroke guidelines and recommendations. Despite consensus among national publications, the utilization of effective and reliable assessment tools does not consistently influence the selection and agreement on outcome measures, which is necessary to advance the field [86][87]. There is a lack of research that conclusively indicates the answer as to whether questionnaires are as effective as measuring physiological properties in post-stroke patients. Many scales and questionnaires consume a large amount of time, require long-term follow-up interviews, and demonstrate low specificity of the impaired function being studied after stroke [88]. It is not uncommon for several people in a team to examine a single patient so that the scale and test lose the reliability of the result [89]. The aim of this review article is to systematize knowledge on the selection of rehabilitation metrics, diagnoses, and upper limb and hand measurements in post-stroke patients. We sought to identify the key parameters in physiotherapy diagnosis responsible for effective rehabilitation. Greater systematization of knowledge exists in the case of upper limb prosthetics, where the range of choices and criteria are extensive and well-defined [90][91][92]. We endeavored to systematize the knowledge of available tools for assessing the upper limb and hand in post-stroke patients, as it is important to determine which scales and tests are suitable for neurological patients. Numerous outcome measures exist, but it is evident that specific scales are often favored, while other, potentially more relevant measures that could reveal the true results of therapy might be overlooked [93].References
- Alt Murphy, M.; Resteghini, C.; Feys, P.; Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015, 15, 29.
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil. Neural Repair. 2017, 31, 793–799.
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702.
- Maceira-Elvira, P.; Popa, T.; Schmid, A.C.; Hummel, F.C. Wearable technology in stroke rehabilitation: Towards improved diagnosis and treatment of upper-limb motor impairment. J. Neuroeng. Rehabil. 2019, 16, 142.
- Rabelo, M.; Nunes, G.S.; da Costa Amante, N.M.; de Noronha, M.; Fachin-Martins, E. Reliability of muscle strength assessment in chronic post-stroke hemiparesis: A systematic review and meta-analysis. Top. Stroke Rehabil. 2016, 23, 26–36.
- Lim, H.; Madhavan, S. Effects of Cross-Education on Neural Adaptations Following Non-Paretic Limb Training in Stroke: A Scoping Review with Implications for Neurorehabilitation. J. Mot. Behav. 2023, 55, 111–124.
- Owen, M.; Ingo, C.; Dewald, J.P.A. Upper Extremity Motor Impairments and Microstructural Changes in Bulbospinal Pathways in Chronic Hemiparetic Stroke. Front. Neurol. 2017, 8, 257.
- Hu, X.; Suresh, A.K.; Rymer, W.Z.; Suresh, N.L. Altered motor unit discharge patterns in paretic muscles of stroke survivors assessed using surface electromyography. J. Neural Eng. 2016, 13, 046025.
- Huang, P.C.; Hsieh, Y.W.; Wang, C.M.; Wu, C.Y.; Huang, S.C.; Lin, K.C. Predictors of motor, daily function, and quality-of-life improvements after upper-extremity robot-assisted rehabilitation in stroke. Am. J. Occup. Ther. 2014, 68, 325–333.
- Sunderland, A. Recovery of ipsilateral dexterity after stroke. Stroke 2000, 31, 430–433.
- Ekstrand, E.; Rylander, L.; Lexell, J.; Brogardh, C. Perceived ability to perform daily hand activities after stroke and associated factors: A cross-sectional study. BMC Neurol. 2016, 16, 208.
- Bertrand, A.M.; Fournier, K.; Wick Brasey, M.G.; Kaiser, M.L.; Frischknecht, R.; Diserens, K. Reliability of maximal grip strength measurements and grip strength recovery following a stroke. J. Hand Ther. 2015, 28, 356–362; quiz 363.
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429.
- Parikh, R.J.; Sutaria, J.M.; Ahsan, M.; Nuhmani, S.; Alghadir, A.H.; Khan, M. Effects of myofascial release with tennis ball on spasticity and motor functions of upper limb in patients with chronic stroke: A randomized controlled trial. Medicine 2022, 101, e29926.
- Plantin, J.; Pennati, G.V.; Roca, P.; Baron, J.C.; Laurencikas, E.; Weber, K.; Godbolt, A.K.; Borg, J.; Lindberg, P.G. Quantitative Assessment of Hand Spasticity After Stroke: Imaging Correlates and Impact on Motor Recovery. Front. Neurol. 2019, 10, 836.
- Dewald, J.P.; Beer, R.F. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 2001, 24, 273–283.
- Reisman, D.S.; Scholz, J.P. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain 2003, 126, 2510–2527.
- Reissner, L.; Fischer, G.; List, R.; Taylor, W.R.; Giovanoli, P.; Calcagni, M. Minimal detectable difference of the finger and wrist range of motion: Comparison of goniometry and 3D motion analysis. J. Orthop. Surg. Res. 2019, 14, 173.
- Du, W.Y.; Huang, T.S.; Hsu, K.C.; Lin, J.J. Measurement of scapular medial border and inferior angle prominence using a novel scapulometer: A reliability and validity study. Musculoskelet. Sci. Pract. 2017, 32, 120–126.
- Mullins, J.; Mawson, C.; Nahavandi, S. Haptic handwriting aid for training and rehabilitation. In Proceedings of the 2005 IEEE International Conference on Systems, Man and Cybernetics, Waikoloa, HI, USA, 12 October 2005; pp. 2690–2694.
- Koter, K.; Samowicz, M.; Redlicka, J.; Zubrycki, I. Hand Measurement System Based on Haptic and Vision Devices towards Post-Stroke Patients. Sensors 2022, 22, 2060.
- Tran, T.Q.B.; du Toit, C.; Padmanabhan, S. Artificial intelligence in healthcare-the road to precision medicine. J. Hosp. Manag. Health Policy 2021, 5, 29.
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788.
- Imura, T.; Nagasawa, Y.; Fukuyama, H.; Imada, N.; Oki, S.; Araki, O. Effect of early and intensive rehabilitation in acute stroke patients: Retrospective pre-/post-comparison in Japanese hospital. Disabil. Rehabil. 2018, 40, 1452–1455.
- Bushnell, C.; Bettger, J.P.; Cockroft, K.M.; Cramer, S.C.; Edelen, M.O.; Hanley, D.; Katzan, I.L.; Mattke, S.; Nilsen, D.M.; Piquado, T.; et al. Chronic Stroke Outcome Measures for Motor Function Intervention Trials: Expert Panel Recommendations. Circ. Cardiovasc. Qual. Outcomes 2015, 8 (Suppl. S3), S163–S169.
- Alt Murphy, M.; Bjorkdahl, A.; Forsberg-Warleby, G.; Persson, C.U. Implementation of evidence-based assessment of upper extremity in stroke rehabilitation: From evidence to clinical practice. J. Rehabil. Med. 2021, 53, jrm00148.
- Wang, L.; Zhu, Q.X.; Zhong, M.H.; Zhou, R.Z.; Liu, X.Q.; Tang, N.S.; Feng, X.C.; Gao, C.F. Effects of corticospinal tract integrity on upper limb motor function recovery in stroke patients treated with repetitive transcranial magnetic stimulation. J. Integr. Neurosci. 2022, 21, 50.
- Xu, Q.; Li, C.; Pan, Y.; Li, W.; Jia, T.; Li, Z.; Ma, D.; Pang, X.; Ji, L. Impact of smart force feedback rehabilitation robot training on upper limb motor function in the subacute stage of stroke. NeuroRehabilitation 2020, 47, 209–215.
- Huang, W.H.; Dou, Z.L.; Jin, H.M.; Cui, Y.; Li, X.; Zeng, Q. The Effectiveness of Music Therapy on Hand Function in Patients With Stroke: A Systematic Review of Randomized Controlled Trials. Front. Neurol. 2021, 12, 641023.
- Krakauer, J.W. Arm function after stroke: From physiology to recovery. Semin. Neurol. 2005, 25, 384–395.
- Chien, W.T.; Chong, Y.Y.; Tse, M.K.; Chien, C.W.; Cheng, H.Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742.
- Hsieh, Y.W.; Wu, C.Y.; Wang, W.E.; Lin, K.C.; Chang, K.C.; Chen, C.C.; Liu, C.T. Bilateral robotic priming before task-oriented approach in subacute stroke rehabilitation: A pilot randomized controlled trial. Clin. Rehabil. 2017, 31, 225–233.
- Yarossi, M.; Patel, J.; Qiu, Q.; Massood, S.; Fluet, G.; Merians, A.; Adamovich, S.; Tunik, E. The Association Between Reorganization of Bilateral M1 Topography and Function in Response to Early Intensive Hand Focused Upper Limb Rehabilitation Following Stroke Is Dependent on Ipsilesional Corticospinal Tract Integrity. Front. Neurol. 2019, 10, 258.
- Prabhakaran, S.; Zarahn, E.; Riley, C.; Speizer, A.; Chong, J.Y.; Lazar, R.M.; Marshall, R.S.; Krakauer, J.W. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil. Neural Repair. 2008, 22, 64–71.
- Teremetz, M.; Colle, F.; Hamdoun, S.; Maier, M.A.; Lindberg, P.G. A novel method for the quantification of key components of manual dexterity after stroke. J. Neuroeng. Rehabil. 2015, 12, 64.
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405.
- Rand, D.; Eng, J.J. Predicting daily use of the affected upper extremity 1 year after stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 274–283.
- Hlustik, P.; Solodkin, A.; Gullapalli, R.P.; Noll, D.C.; Small, S.L. Somatotopy in human primary motor and somatosensory hand representations revisited. Cereb. Cortex 2001, 11, 312–321.
- Hamzei, F.; Dettmers, C.; Rijntjes, M.; Weiller, C. The effect of cortico-spinal tract damage on primary sensorimotor cortex activation after rehabilitation therapy. Exp. Brain Res. 2008, 190, 329–336.
- Wang, H.; Arceo, R.; Chen, S.; Ding, L.; Jia, J.; Yao, J. Effectiveness of interventions to improve hand motor function in individuals with moderate to severe stroke: A systematic review protocol. BMJ Open 2019, 9, e032413.
- Gowda, A.S.; Memon, A.N.; Bidika, E.; Salib, M.; Rallabhandi, B.; Fayyaz, H. Investigating the viability of motor imagery as a physical rehabilitation treatment for patients with stroke-induced motor cortical damage. Cureus 2021, 13, e14001.
- Wilkins, K.B.; Owen, M.; Ingo, C.; Carmona, C.; Dewald, J.P.A.; Yao, J. Neural Plasticity in Moderate to Severe Chronic Stroke Following a Device-Assisted Task-Specific Arm/Hand Intervention. Front. Neurol. 2017, 8, 284.
- Lan, Y.; Yao, J.; Dewald, J.P.A. The Impact of Shoulder Abduction Loading on Volitional Hand Opening and Grasping in Chronic Hemiparetic Stroke. Neurorehabil. Neural Repair. 2017, 31, 521–529.
- Smania, N.; Paolucci, S.; Tinazzi, M.; Borghero, A.; Manganotti, P.; Fiaschi, A.; Moretto, G.; Bovi, P.; Gambarin, M. Active finger extension: A simple movement predicting recovery of arm function in patients with acute stroke. Stroke 2007, 38, 1088–1090.
- Nijland, R.H.; van Wegen, E.E.; Harmeling-van der Wel, B.C.; Kwakkel, G. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: Early prediction of functional outcome after stroke: The EPOS cohort study. Stroke 2010, 41, 745–750.
- Boake, C.; Noser, E.A.; Ro, T.; Baraniuk, S.; Gaber, M.; Johnson, R.; Salmeron, E.T.; Tran, T.M.; Lai, J.M.; Taub, E.; et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil. Neural Repair. 2007, 21, 14–24.
- Lang, C.E.; DeJong, S.L.; Beebe, J.A. Recovery of thumb and finger extension and its relation to grasp performance after stroke. J. Neurophysiol. 2009, 102, 451–459.
- Stinear, C.M.; Barber, P.A.; Petoe, M.; Anwar, S.; Byblow, W.D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012, 135 Pt 8, 2527–2535.
- Carpinella, I.; Jonsdottir, J.; Ferrarin, M. Multi-finger coordination in healthy subjects and stroke patients: A mathematical modelling approach. J. Neuroeng. Rehabil. 2011, 8, 19.
- Wenzelburger, R.; Kopper, F.; Frenzel, A.; Stolze, H.; Klebe, S.; Brossmann, A.; Kuhtz-Buschbeck, J.; Golge, M.; Illert, M.; Deuschl, G. Hand coordination following capsular stroke. Brain 2005, 128 Pt 1, 64–74.
- Ewoldt, J.K.; Raghavan, P.; Suresh, N.L. Quantitative Measurement of Resistance to Passive Joint Motion in Chronic Stroke Survivors. In Spasticity and Muscle Stiffness: Restoring Form and Function; Springer: Berlin/Heidelberg, Germany, 2022; pp. 47–62.
- Hu, X.L.; Tong, K.Y.; Li, L. The mechanomyography of persons after stroke during isometric voluntary contractions. J. Electromyogr. Kinesiol. 2007, 17, 473–483.
- O’Dwyer, N.J.; Ada, L.; Neilson, P.D. Spasticity and muscle contracture following stroke. Brain 1996, 119 Pt 5, 1737–1749.
- Kamper, D.G.; Harvey, R.L.; Suresh, S.; Rymer, W.Z. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve 2003, 28, 309–318.
- Kamper, D.G.; Fischer, H.C.; Cruz, E.G.; Rymer, W.Z. Weakness is the primary contributor to finger impairment in chronic stroke. Arch. Phys. Med. Rehabil. 2006, 87, 1262–1269.
- Singh, N.; Saini, M.; Kumar, N.; Srivastava, M.V.P.; Mehndiratta, A. Evidence of neuroplasticity with robotic hand exoskeleton for post-stroke rehabilitation: A randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 76.
- Alfano, C.M.; Zucker, D.S.; Pergolotti, M.; Ness, K.K.; Jones, L.W.; Price, N.D.; Schmitz, K.H.; Ligibel, J.A. A precision medicine approach to improve cancer rehabilitation’s impact and integration with cancer care and optimize patient wellness. Curr. Phys. Med. Rehabil. Rep. 2017, 5, 64–73.
- Oh, B.M. A Path to Precision Medicine: Incorporating Blood-Based Biomarkers in Stroke Rehabilitation. Ann. Rehabil. Med. 2021, 45, 341–344.
- Chen, Q.; Cao, C.; Gong, L.; Zhang, Y. Health related quality of life in stroke patients and risk factors associated with patients for return to work. Medicine 2019, 98, e15130.
- Fatema, Z.; Sigamani, A.; Vikneswaran, G.; Manuel, D. ‘Quality of life at 90 days after stroke and its correlation to activities of daily living’: A prospective cohort study. J. Stroke Cerebrovasc. Dis. 2022, 31, 106806.
- Baker, K.; Cano, S.J.; Playford, E.D. Outcome measurement in stroke: A scale selection strategy. Stroke 2011, 42, 1787–1794.
- Cieza, A.; Fayed, N.; Bickenbach, J.; Prodinger, B. Refinements of the ICF Linking Rules to strengthen their potential for establishing comparability of health information. Disabil. Rehabil. 2019, 41, 574–583.
- Stucki, G.; Ewert, T.; Cieza, A. Value and application of the ICF in rehabilitation medicine. Disabil. Rehabil. 2002, 24, 932–938.
- Lemmens, R.J.; Timmermans, A.A.; Janssen-Potten, Y.J.; Smeets, R.J.; Seelen, H.A. Valid and reliable instruments for arm-hand assessment at ICF activity level in persons with hemiplegia: A systematic review. BMC Neurol. 2012, 12, 21.
- Cantero-Tellez, R.; Naughton, N.; Algar, L.; Valdes, K. Outcome measurement of hand function following mirror therapy for stroke rehabilitation: A systematic review. J. Hand Ther. 2019, 32, 277–291.e1.
- Van Gils, A.; Meyer, S.; Van Dijk, M.; Thijs, L.; Michielsen, M.; Lafosse, C.; Truyens, V.; Oostra, K.; Peeters, A.; Thijs, V.; et al. The Adult Assisting Hand Assessment Stroke: Psychometric Properties of an Observation-Based Bimanual Upper Limb Performance Measurement. Arch. Phys. Med. Rehabil. 2018, 99, 2513–2522.
- Santisteban, L.; Teremetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792.
- Poole, J.L.; Whitney, S.L. Assessments of motor function post stroke: A review. Phys. Occup. Ther. Geriatr. 2001, 19, 1–22.
- Harrison, J.K.; McArthur, K.S.; Quinn, T.J. Assessment scales in stroke: Clinimetric and clinical considerations. Clin. Interv. Aging 2013, 8, 201–211.
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair. 2002, 16, 232–240.
- Hope, T.M.; Friston, K.; Price, C.J.; Leff, A.P.; Rotshtein, P.; Bowman, H. Recovery after Stroke: Not So Proportional after All? Oxford University Press: Oxford, UK, 2019.
- Quinn, T.; Dawson, J.; Walters, M.; Lees, K. Functional outcome measures in contemporary stroke trials. Int. J. Stroke 2009, 4, 200–205.
- Burke, J.W.; McNeill, M.; Charles, D.; Morrow, P.; Crosbie, J.; McDonough, S. Serious games for upper limb rehabilitation following stroke. In Proceedings of the 2009 Conference in Games and Virtual Worlds for Serious Applications, Coventry, UK, 23–24 March 2009; pp. 103–110.
- Meng, L.; Jiang, X.; Qin, H.; Fan, J.; Zeng, Z.; Chen, C.; Zhang, A.; Dai, C.; Wu, X.; Akay, Y.M.; et al. Automatic Upper-Limb Brunnstrom Recovery Stage Evaluation via Daily Activity Monitoring. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2589–2599.
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375.
- Nam, K.E.; Lim, S.H.; Kim, J.S.; Hong, B.Y.; Jung, H.Y.; Lee, J.K.; Yoo, S.D.; Pyun, S.-B.; Lee, K.M.; Lee, K.J. When does spasticity in the upper limb develop after a first stroke? A nationwide observational study on 861 stroke patients. J. Clin. Neurosci. 2019, 66, 144–148.
- Daly, J.J.; Ruff, R.L.; Osman, S.; Hull, J.J. Response of prolonged flaccid paralysis to FNS rehabilitation techniques. Disabil. Rehabil. 2000, 22, 565–573.
- Lin, Y.F.; Liu, X.H.; Cui, Z.Y.; Song, Z.T.; Zou, F.; Chen, S.G.; Kang, X.Y.; Ye, B.; Wang, Q.; Tian, J.; et al. Weakened Effective Connectivity Related to Electroacupuncture in Stroke Patients with Prolonged Flaccid Paralysis: An EEG Pilot Study. Neural Plast. 2021, 2021, 6641506.
- Correa-Forero, V.; Pinilla-Monsalve, G.D.; Valderrama-Chaparro, J.A.; Amaya-Gonzalez, P. Cryptococcal meningitis presenting as acute flaccid paralysis: A case report. J. Infect. Public Health 2020, 13, 143–148.
- Ramroop, H.; Cruz, R. Electrodiagnostic Evaluation of Motor Neuron Disease; StatPearls Publishing: Treasure Island, FL, USA, 2020.
- Ivanhoe, C.B.; Reistetter, T.A. Spasticity: The misunderstood part of the upper motor neuron syndrome. Am. J. Phys. Med. Rehabil. 2004, 83 (Suppl. S10), S3–S9.
- Lackritz, H.; Parmet, Y.; Frenkel-Toledo, S.; Banina, M.C.; Soroker, N.; Solomon, J.M.; Liebermann, D.G.; Levin, M.F.; Berman, S. Effect of post-stroke spasticity on voluntary movement of the upper limb. J. Neuroeng. Rehabil. 2021, 18, 81.
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990–2010: Findings from the global burden of disease study 2010. Lancet 2014, 383, 245–254.
- Borges, L.R.; Fernandes, A.B.; Oliveira Dos Passos, J.; Rego, I.A.O.; Campos, T.F. Action observation for upper limb rehabilitation after stroke. Cochrane Database Syst. Rev. 2022, 8, CD011887.
- Gregor, S.; Saumur, T.M.; Crosby, L.D.; Powers, J.; Patterson, K.K. Study Paradigms and Principles Investigated in Motor Learning Research After Stroke: A Scoping Review. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100111.
- Miller, E.L.; Murray, L.; Richards, L.; Zorowitz, R.D.; Bakas, T.; Clark, P.; Billinger, S.A.; American Heart Association Council on Cardiovascular Nursing and the Stroke Council. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the American Heart Association. Stroke 2010, 41, 2402–2448.
- Prange-Lasonder, G.B.; Alt Murphy, M.; Lamers, I.; Hughes, A.M.; Buurke, J.H.; Feys, P.; Keller, T.; Klamroth-Marganska, V.; Tarkka, I.M.; Timmermans, A.; et al. European evidence-based recommendations for clinical assessment of upper limb in neurorehabilitation (CAULIN): Data synthesis from systematic reviews, clinical practice guidelines and expert consensus. J. Neuroeng. Rehabil. 2021, 18, 162.
- Chalos, V.; van der Ende, N.A.M.; Lingsma, H.F.; Mulder, M.; Venema, E.; Dijkland, S.A.; Berkhemer, O.A.; Yoo, A.J.; Broderick, J.P.; Palesch, Y.Y.; et al. National Institutes of Health Stroke Scale: An Alternative Primary Outcome Measure for Trials of Acute Treatment for Ischemic Stroke. Stroke 2020, 51, 282–290.
- Vidmar, T.; Goljar Kregar, N.; Puh, U. Reliability of the Modified Ashworth Scale After Stroke for 13 Muscle Groups. Arch. Phys. Med. Rehabil. 2023, 104, 1606–1611.
- Resnik, L.; Adams, L.; Borgia, M.; Delikat, J.; Disla, R.; Ebner, C.; Walters, L.S. Development and evaluation of the activities measure for upper limb amputees. Arch. Phys. Med. Rehabil. 2013, 94, 488–494.e4.
- Wright, V. Prosthetic outcome measures for use with upper limb amputees: A systematic review of the peer-reviewed literature, 1970 to 2009. JPO J. Prosthet. Orthot. 2009, 21, P3–P63.
- Lindner, H.Y.; Natterlund, B.S.; Hermansson, L.M. Upper limb prosthetic outcome measures: Review and content comparison based on International Classification of Functioning, Disability and Health. Prosthet. Orthot. Int. 2010, 34, 109–128.
- Swingler, G.H.; Volmink, J.; Ioannidis, J.P. Number of published systematic reviews and global burden of disease: Database analysis. BMJ 2003, 327, 1083–1084.
More
