Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nariman Shahhosseini and Version 2 by Fanny Huang.
Mosquito-borne diseases (MBDs) have global public health implications to both humans and animals, making it a One Health priority concern. Ongoing climatic change creates favourable conditions for the emergence of exotic MBDs in previously disease-free areas.
- arboviruses
- climate change
- mosquito
- Iran
1. Introduction
Emerging and re-emerging vector-borne diseases are a major global concern, straining healthcare systems, hindering economic and social development both locally and on a global scale [1][2][1,2]. Vector-borne diseases (VBDs) represent more than 17% of all infectious diseases, which the World Health Organization (WHO) has estimated to cause over 700,000 deaths annually [3]. VBDs are increasing at an alarming rate with a disproportionate number of new illnesses being caused by viruses [1]. In fact, many viral hemorrhagic fevers (VHF) are vector-borne viral diseases (VBVDs) that transmit highly pathogenic agents from arthropods to humans and are responsible for deadly intercontinental outbreaks [4][5][4,5]. VBVDs are affected by a variety of peripheral factors, whether human, animal, or environmental (Figure 1), including climate change, urbanization, and travel.
VBVDs are spread by many vectors, including blood-sucking arthropods (e.g., mosquitoes, ticks, sandflies, and biting midges). Of the pathogens responsible for VBVDs, arboviruses cause countless animal and human infections worldwide. Recent data indicated that arthropod vectors such as mosquitoes or ticks, can carry more than one pathogen, leading to the co-transmission of diseases through one bite. This highlights the importance of VBVDs as a concern to public health [6]. Furthermore, while VBVDs are transmitted by a main vector, secondary vectors such as rodents often can transmit the same pathogens as insects, leading to multiple routes for infection [7].
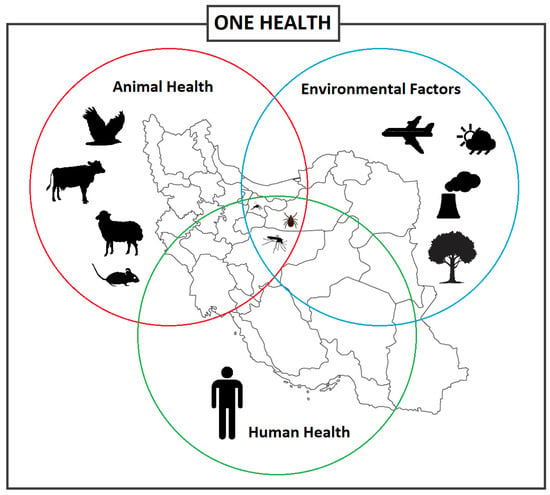
Figure 1. Different factors and interactions between the vector-borne diseases, vectors, and hosts including animals and humans, and environmental factors, informing the One Health concept.
A major factor influencing living and circulation patterns of pathogens and vectors are biospheric changes, particularly those involving climate systems. Ongoing climate change is affecting shifts in ecosystems and agricultural production systems [8], which in turn, alter VBVD transmission and incidence patterns [9]. Global warming has known correlations with increased development, survival, density, and expansion of ticks and mosquitoes. Furthermore, extreme weather events, such as floods, triggered by climate change, frequently have a sustaining effect on vector density. Countries in the Middle East are considered to be especially susceptible to the effects of climate change and the ensuing alterations of wet and dry climate bands [10]. In fact, within the next decades, temperatures in Iran are expected to rise 2.6 °C with a decline in precipitation, which can be anticipated to affect VBVD patterns.
The climatic changes effecting the Middle East are pronounced in Iran due to its vast geographical area, with various terrain and different climatic classification zones [2]. The country is home to various vectors and hosts and has the potential to be a major zone for VBVD outbreaks, as both Iran and many of its surrounding countries are endemic for VBVDs. Iran itself is endemic for several medically important VBVDs, such as Crimean–Congo hemorrhagic fever (CCHF) [11][12][11,12].
Several vectors of VBVDs including mosquitoes, ticks, sandflies, and rodents have been identified and are known to inhabit the natural fauna of Iran. Mosquitoes are known to be vectors for several medically significant public health VBVDs [13][14][13,14] including Chikungunya virus (CHIKV) [15], Dengue virus (DENV) [16], and West Nile virus (WNV) [17]. Iran is home to many different mosquito species, with 70 different species reported thus far [14][18][14,18]. Mosquitoes can survive and propagate under various climatic conditions [19]; therefore, increasing temperatures due to climate change is projected to have an extreme effect on distribution dynamics of infectious diseases [20]. Driving the increase in global distribution of disease is alterations to the expected ranges of mosquitoes by expanding populations to northern territories as temperatures rise or by broadening seasonal viability due to longer periods of warm weather.
2. Mosquito-Borne Viruses in Iran
2.1. Chikungunya Virus
Chikungunya virus (CHIKV), a mosquito borne virus (MBV), is a re-emerging Alphavirus that belongs to the Togaviridae family [21][22][34,35] and is deemed a worldwide threat that has caused epidemics in several countries [23][36]. Climate change, increased international trade, and increased travel are facilitating the local and global spread of the viruses. Viremic travelers can introduce the virus into regions where competent vectors are found, leading to potential autochthonous transmission, creating regions endemic for the disease, as demonstrated during the CHIKV outbreak in Italy [24][37].
CHIKV originated in Africa and was first detected in 1952 in Tanzania and isolated in 1953 [25][26][38,39]. The virus is grouped into 3 distinct lineages: West African, Asian, and East/Central/South/Africa [21][34]. CHIKV is a positive-sense single stranded RNA virus of approximately 11.8 kb with two open reading frames (ORFs). The initial ORF encodes four non-structural proteins and the other ORF encodes five structural proteins, which includes the capsid, as well as an envelope that is made of four proteins [21][23][24][27][34,36,37,40].
CHIKV infection is categorized as an acute febrile illness with an incubation period of four to seven days, similar to Dengue, Zika, and Malaria, leading to possible misdiagnosis, especially in the case of co-infections with other febrile illnesses [28][41]. Symptoms of Dengue are similar to CHIKV infection, making diagnosis challenging [29][42]. Patients with symptoms present with fever, severe arthralgia, skin rash, headaches, muscle aches, and joint pain [23][29][36,42]. CHIKV infection is seldom fatal in humans and in many cases resolves within 3 weeks; however, severe cases (10–15%) can lead to debilitating pain that can last weeks, if not years. Apart from being mosquito-borne, vertical transmission (mother to child) has been documented [29][42]. CHIKV is diagnosed through viral isolation using cell cultures, serological tests using antibodies, and RT-PCR tests using nucleic acids [21][34].
2.1.1. Chikungunya Virus Situation in Iran
The first report of CHIKV circulation in the Middle East was recorded in Pakistan (1981), with recurring outbreaks in Pakistan, Saudi Arabia, and Yemen [30][31][32][43,44,45]. CHIKV is assumed to be present in Iran due to the presence of the virus in neighboring countries such as Pakistan [23][33][36,46]. During the 2017 outbreak in Pakistan, travel from Pakistan likely introduced the virus to Iran [23][33][36,46]. Several studies have been undertaken to detect the virus in Iran. Pouriayevali et al., 2019, examined serum from 159 patients with suspected CHIKV illness in the Iranian province of Sistan and Baluchestan, which borders Pakistan [23][36]. The study revealed that 25.1% of the serum samples were positive for CHIKV. A similar study by Tavakoli et al., 2020, examined serum from patients from 7 different provinces (Khuzestan, Fars, Kerman, Ilam, Hormozgan, Bushehr, Sistan and Baluchestan) with 16.07% of the samples testing positive for CHIKV [15]. Another study in 2018 examining blood samples collected from children at a children’s hospital in Tehran identified 2.2% positivity rate for CHIKV [34][47]. Similarly, serum samples collected in six provinces (Bushehr, Hormozgan, Sistan and Baluchestan, Khuzestan, Gilan and Mazandaran) from 2017 to 2018 revealed that 1.8% of the samples tested positive for CHIKV [35][48]. Altogether, these studies provide strong evidence of circulation of the virus in Iran.
2.1.2. Chikungunya Virus Vectors in Iran
Reports of CHIKV outbreaks in the countries neighboring Iran, such as Pakistan, Qatar, Yemen, Iraq, Turkey, and Saudi Arabia suggest an existential threat toward the emergence of CHIKV in Iran. Viremic individuals are a major source of CHIKV dissemination, which can further extend locally through the spreading of infected mosquitoes. The main CHIKV vectors are Aedes aegypti and Aedes albopictus, the latter being confirmed in the south-eastern regions of Iran bordering Pakistan [36][49]. Mosquitoes maintain the ‘sylvatic cycle’ in monkeys and other vertebrates as common reservoirs for the virus. CHIKV is similar to other arboviruses, such as Dengue virus (DENV) and Zika virus (ZIKV), which are transmitted via the urban transmission cycle between humans and mosquitoes [23][36]. Recently, an Iranian study undertaken in the Mazandaran, North Khorasan, and Fars provinces aimed at detecting arboviruses in mosquitoes identified CHIKV (Asian genotype) in 3 different mosquito species [33][46]. Interestingly, the three species identified to carry CHIKV, Anopheles maculipennis s.l., Culiseta longiareolata, and Culex tritaeniorhynchus are not the main vectors for CHIKV. Furthermore, it was the first report of infection of CHIKV in An. maculipennis s.l., and Cx. tritaeniorhynchus. Identification of new potential vectors and the presence of the main vectors for CHIKV in Iran suggests an increased risk for CHIKV outbreak in Iran.
2.2. Dengue Virus
Dengue fever (DF) is an MBV that is considered a major global threat [37][50]. Its causative agent, DENV, is the principal source of VHF infections worldwide, causing Dengue hemorrhagic fever (DHF) [38][51]. DF incidence has grown significantly over the past 20 years, with the number of cases reported to the WHO dramatically increasing from ~500,000 cases in 2000 to 5.2 million cases in 2019, with the death toll quadrupling in the last four years, from 960 in 2015 to 4032 in 2019 [39][40][52,53]. As the result of biological, environmental, and socioeconomic factors such as climate change, urbanization, and international travel, DENV has expanded to areas previously free of the virus [41][54]. An estimated 3.9 billion people live in Dengue-endemic countries, including Afghanistan and Pakistan, which share an international border with Iran.
DENV belongs to the family of Flaviviridae and genus Flavivirus and is an 11-kb single-stranded positive-sense RNA virus [37][41][42][50,54,55]. The genome encodes a polyprotein that is composed of 3 structural and 7 non-structural proteins [37][41][42][50,54,55] and is classified into 4 serotypes (DENV1–4) [43][56]. Dengue has a 4–10 day incubation with a wide spectrum of clinical signs and symptoms, often with unpredictable clinical outcomes. All four virus serotypes can cause febrile illness [44][57]. While the infection is self-limiting in the majority of cases and patients fully recover from mild symptoms such as fever, headache, rashes, and muscle/joint pain [37][50], a small percentage of patients develop severe diseases, mostly characterized by severe abdominal pain, cardiac/pulmonary/hepatic problems, and plasma leakage with or without hemorrhagic syndrome (DF or DHF). The hemorrhagic form can be easily misdiagnosed as another hemorrhagic disease. DENV can be diagnosed using ELISA tests (detection of antibodies/antigens), virus isolation, or RT-PCR tests, and DENV antibodies can be present for 2–3 months after infection [37][44][45][50,57,58].
2.2.1. Dengue Virus Situation in Iran
Neighboring countries sharing a border with Iran, such as Afghanistan and Pakistan, are endemic for DENV, potentiating the spread into Iran [46][59]. DENV became a public health concern in Iran in 2008, after the first case of Dengue fever was reported in a patient who had previously travelled to Malaysia [47][60]. Similarly, two Iranians with confirmed DENV had a history of travel to Malaysia, during years of comparably high-infection rates (2009 and 2011, respectively) [48][61]. Another imported case of DENV into Iran was reported in 2015, when a patient with recent history of travel to India was admitted to a hospital with signs and symptoms of the disease and subsequently tested positive for DENV 2, genotype 4, similar to the strain isolated from patients in India [16]. These studies provide evidence on how international travel can have an impact on disease spread from endemic countries.
In a retrograde investigation in Iran, the presence of specific antibodies against DENV was observed in 5% of a previously studied population. Among the seropositive cases, 53% of the population had a travel history to Dengue endemic countries including Malaysia, India, and Thailand; conversely, 46% of the population did not [49][62]. However, those individuals with no travel history reside in Iranian provinces that share a border with either Pakistan (Sistan and Baluchestan province) or Iraq (Kurdistan province), suggesting the presence of DENV in Iran was due to proximity to endemic countries. Another study searching for the incidence of DENV among the healthy population in Chabahar city in south-eastern Iran showed ~6% of the studied population were seropositive for DENV [50][63]. A similar study on sera from patients presenting with rash and fever collected during 2016 and 2017 showed that 6.27% of the studied population were DENV seropositive [15]. Lastly, a study by Heydari et al., 2018, aimed to determine if serum samples of patients presenting with symptoms of DENV but negative for CCHF from 2013 to 2015 in the province of Sistan and Baluchestan, which borders Pakistan, were positive for DENV [45][58]. The study determined that 13 of the 60 patients were exposed to DENV, providing support that DENV is circulating in Southeastern Iran. Altogether, these results provide strong evidence that DENV is circulating in Iran, especially in provinces that border countries endemic for DENV.
2.2.2. Dengue Virus Vectors in Iran
Ae. aegypti and Ae. albopictus are the two main vectors of DENV, which are endemic to tropical and subtropical climates. DENVs circulate between humans and vector mosquitoes with no intermediate host. Thus, the spatial distribution of the vectors highly affects the epidemiology of the disease [37][46][51][50,59,64]. The presence of Ae. aegypti was recently confirmed both morphologically and molecularly in southern Iran [52][65], and Ae. albopictus has been isolated both in 2009 and 2013 in Iran [36][49]. The Ae. albopictus mosquito is considered a conspicuously menacing and versatile species that inhabits both temperate and tropical climate regions. Another species, Aedes unilineatus, was identified also in the south-east of Iran (2012–2014) and, interestingly, has been previously reported to be the DENV vector in Karachi, Pakistan [53][66]. The presence of these mosquito vectors in the regions of Iran where previous seropositive human cases for DENV with no travel history was reported, indicates the risk of DENV outbreaks exists with the potential for autochthonous transmission to humans in this area.
2.3. Sindbis Virus
Sindbis fever is an illness transmitted by mosquitoes infected with the Sindbis virus (SINV) [54][67]. First isolated from mosquitoes in 1952 in Egypt, SINV belongs to the family Togaviridae and genus Alphavirus [21][54][34,67]. The virus is enveloped and is made of positive-sense, single-stranded RNA genome with a genome size of 11.7 kb that encodes four non-structural and five structural proteins [55][68]. SINV can be grouped into six different genotypes. SINV- I, which is associated with human outbreaks, has been isolated from the Middle East, among other countries.
The virus is widely distributed in Africa, Asia, Eurasia, and Oceania with sporadic outbreaks in Australia, China, and South Africa [56][69]. Genetic studies have showed that SINV strains isolated in Africa, Europe, and the Middle East are geographically distinct serotypes [57][70]. Despite SINV being identified across various continents, clinical infections are mainly reported in Europe and Africa [58][71]. Signs and symptoms of SINV include fever, rash, arthritis, and myalgia [58][71], which in most cases resolves within a few weeks [59][72]. However, in some cases, the arthritis and myalgia can persist for years. The chronic disease syndrome following SINV infection shares many features with autoimmune diseases, which is thought to be immune mediated [59][72]. SINV is typically identified using specific antibodies in human serum samples, as virus isolation has seldom been used successfully to identify SINV [58][60][71,73]. Antibodies can persist for several months post infection [58][71].
2.3.1. Sindbis Virus Situation in Iran
SINV is a zoonotic disease transmitted from birds to humans via mosquitoes, which may spread to non-endemic countries by migratory birds [58][61][71,74]. A study undertaken by Hanafi-Bojd et al., 2021, identified SINV in both Culex pipiens complex and Culex theileri in the West Azerbaijan province of Iran [62][75], with both mosquito species distributed across the country. Furthermore, these mosquito habitats are suitable also for the migratory birds participating in the zoonotic cycle of SINV. Altogether, these results strongly suggest the virus has a high potential to spread to humans in the area, though, thus far, only two human cases of SINV have been reported in Iran.
2.3.2. Sindbis Virus Vectors in Iran
Interestingly, SINV mosquito vectors are different than that of CHIKV and DENV, as SINV is mainly transmitted by Culex mosquitoes [55][61][68,74]. In 1952, SINV was first isolated from Cx. pipiens and Cx. univittatus [63][76]. Over the years it has been determined that the Aedes, Culiseta, and Culex mosquito species are all viral vectors that help maintain the transmission cycle between wild birds and humans. In Iran, the mosquitoes that transmit SINV are readily identified throughout the country, which is a cause for concern, making monitoring of the disease and its vectors of crucial importance [62][64][65][75,77,78]. Furthermore, studies show that SINV is transmitted from Culex females to their offspring, facilitating disease maintenance [66][79].
2.4. West Nile Virus
West Nile virus (WNV) is a zoonotic flavivirus that is part of the Japanese encephalitis virus complex [67][68][80,81]. It is both a human and animal pathogen, notably infecting birds and horses. WNV has global public health implications to both humans and animals, making it a One Health priority concern [68][69][81,82]. WNV is a single-stranded RNA virus of approximately 11 kb and contains a single ORF that encodes three structural and seven non-structural proteins [67][68][80,81]. WNV is grouped in eight distinct lineages. Lineage 1 consists of two sub-lineages: clade 1A is formed by widespread strains and clade 1B by Australian WNV Kunjin strains. Lineage 2 is distributed in Sub-Saharan Africa and Europe. Lineage 3 circulates in the Czech Republic. Lineage 4 was detected from the Caucasus. Lineage 5 contains a WNV isolate from India [70][83]. Lineage 6 contains a Malaysian Kunjin virus [71][84]. The African Koutango virus is closely related to WNV and considered lineage 7. WNV lineage 8 has been reported from Spain [72][73][85,86]. Finally, lineages 1, 2, and 5 are known to cause human infection [74][87].
WNV was first detected in 1937 in the blood of a febrile woman in the West Nile region of north-western Uganda [75][88]. Over the past few years, several outbreaks have occurred in Europe [76][89], including a large outbreak with over 2000 cases in 2018 [77][90]. The incubation period for WNV infection in humans ranges from 3–15 days [78][79][91,92]. Most infected humans (75–80%) have very mild signs and symptoms of the disease and are either asymptomatic or have mild fever. Approximately, 20% of infected humans have fever and flu-like symptoms, and less than 1% of infected humans have neuroinvasive complications [68][74][81,87]. Neuroinvasive disease is characterized by meningitis, encephalitis, and movement disorders that can have lifelong implications [68][81]. WNV is typically detected using diagnostic approaches including virus isolation, RT-PCR, serological assays using antibodies, and pathological tests examining tissues microscopically.
2.4.1. West Nile Virus Situation in Iran
Human cases of WNV occur globally, including in Iran [80][93]. The first human cases of WNV in Iran were detected in 1976 in the central and southwestern regions of Iran [81][94]. Since then, various diagnostic studies have been undertaken to identify WNV in Iran. Two serological surveys occurred in the 2000s, one that determined 5% of healthy blood donors in Tehran city were serologically positive for WNV [82][95]. The second identified that 15% of migratory/resident wild birds were seropositive for WNV in north-western Iran [83][96]. WNV was identified as the causative agent for encephalitic signs in the cerebrospinal fluid (CSF) of Iranian patients in Isfahan city in 2008 and 2009 [84][97]. Subsequently, this strain was determined to be genetically related to the lineage 2 Central African Republic WNV strain [85][98].
Studies published in the last 10 years are still identifying substantial WNV infections in Iran. Serological studies undertaken from 2010 to 2012 have demonstrated that WNV was observed in 1.3% of human samples and 2.8% of equine samples from five provinces in North and Central Iran [86][99]. Additionally, 11% of the investigated population residing in Mashhad city in north-eastern Iran were positive from previous exposure to WNV [80][93]. A study examining blood donors in the Iranian province of Sistan and Baluchestan identified WNV in 8.24% of blood donors, revealing the circulation of the disease in the human population in the province [87][100]. Another study by Ziyaeyan et al., 2018, in the Hormozgan province that examined human serum samples and mosquitoes for WNV revealed that 20.6% of the human samples were positive for WNV [88][101]. The virus was also identified in Cx. pipiens pools in the region, providing evidence of virus circulation in the province. Lastly, a study examining serum samples from birds and horses in 4 provinces: Kordestan, Mazandaran, Golestan, and North Khorasan identified WNV in ~14% of birds and ~17% of horses, indicating the presence of the virus in North Iran [17]. Altogether, these results provide evidence for the continuous presence and circulation of WNV in Iran, indicating a need for strong monitoring and protocols to minimize the potential of a large-scale outbreak in the country.
2.4.2. West Nile Virus Vector in Iran
The primary mosquito vectors for WNV are the Culex mosquitoes. A large-scale study was undertaken to examine mosquitoes for the presence of arboviruses (including WNV). The study reported WNV lineage-2 in Cx. pipiens pipiens form pipiens (Cpp) in the province of Gilan [89][90][102,103]. Of note, the presence of WNV lineage 2 in Cpp and the major abundancy of this species in parts of Iran [91][92][104,105] lends support to this vector being responsible for both enzootic and epizootic transmission of WNV [67][80]. Another study identified WNV in an Aedes caspius mosquito species [83][96], and subsequent studies identified these as lineage 1 WNV strains [93][106], suggesting co-circulation of WNV lineages in Iran. In addition, Shahhosseini et al. identified WNV in Cx. theileri, one of the most common mosquito species in Iran, which suggests this may be a new vector for WNV within the country [94][107]. Furthermore, a recent study in southern Iran provided evidence that WNV can be transmitted vertically between female Culex mosquitoes and their offspring, which may explain how the virus survives the winter months. This consideration expands concerns beyond an enzootic seasonal consideration, making WNV an even greater public health and One Health concern in Iran [95][108].
2.5. Other Related Mosquito-Borne Viruses in Iran
2.5.1. Rift Valley Fever Virus Situation in Iran
Rift Valley Fever virus (RVFV) is a mosquito-borne Phlebovirus that mainly affects ruminants but can also infect humans [96][97][109,110]. The primary vectors for RVFV are the Aedes mosquitoes but Culex, Anopheles, and Mansonia are secondary vectors [98][111], all of which have been identified in Iran [14][18][14,18]. RVFV disease in humans vary from a mild febrile illness that resolves in a few days to acute forms causing severe signs and symptoms such as blindness, encephalitis, and death [97][98][110,111].
RVFV is widespread in Africa, with spillover to the Comoros Archipelago (including Mayotte), Madagascar, Saudi Arabia, and Yemen [99][112]. Following the outbreak of RVF in Saudi Arabia in 2000, a surveillance of both animal and human populations was conducted in neighboring Iran from 2001 to 2011. While human cases of RVFV have not been identified in Iran [100][113], the virus antibody (IgG) has been identified in cattle and sheep in the province of Kurdistan [96][109]. Recently, another investigation to study associated risk factors in aborted sheep in Kurdistan province showed that 1.65% of fetal abortions in sheep may be linked to seropositivity to RVFV [96][109]. Furthermore, sandflies and tabanids, which are present in Iran, have been shown to participate in virus transmission, increasing the potential vectors for RVFV [101][102][114,115]. Thus far, although direct evidence of RVFV circulation in Iran is lacking, continued enhanced surveillance remains a One Health concern toward revealing the real status of RVFV in Iran and preventing a potential spillover of RFVF to humans there.
2.5.2. Zika Virus Situation in Iran
Zika virus (ZIKV) is a mosquito-borne Flavivirus that gained notoriety in 2015 when it spread from South America to North America [103][116]. ZIKV infection is not fatal but can cause serious complications, especially for children born from infected mothers [104][117]. Presently, ZIKV has not been identified in Iran; still, surveillance has been enhanced, especially due to a potential for travel-related cases of the disease [103][104][105][116,117,118]. A study in 2018 examining both human serum and mosquitoes in southern Iran did not find ZIKV, and the main vectors, Ae. aegypti and Ae. albopictus, were not identified in the mosquito samples [88][101]. Recently, however, a main ZIKV vector, Ae. aegypti was identified in southern Iran [52][65]. While ZIKV has not been identified, the presence of the vector is a cause for concern and surveillance should be continued to avoid a potential future outbreak. More studies examining ZIKV vectors need to be undertaken to elucidate the potential of disease spread in Iran, especially considering the presence of a main vector for the disease.
