Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ajay K Dalai and Version 2 by Rita Xu.
There is a growing interest in the production of biofuels and biochemicals from renewable biomass. Biomass in the form of woody and agricultural residues, municipal solid waste and other organic refuse is becoming popular as a feedstock for biofuel and biochemical production through thermochemical and biological routes. Methanol, a widely used industrial chemical, also has clean fuel properties due to its high-octane number, low flammability, low emissions and high engine performance.
- biomass
- methanol
- techno-economic analysis
1. Introduction
Methanol production has wide applications for electricity generation and hydrogen transportation. In addition, methanol has many applications including dimethyl carbonate and dimethyl ether production [1]. Recently, methanol production from syngas through catalytic Fischer-Tropsch synthesis, which is not fully carbon-neutral, has raised environmental concerns. Production of methanol from biomass generates many benefits such as sustainability and commercial viability [2]. Green methanol can be generated from different bio-based resources and clean technologies including gasification, reforming, stripper-off gas and power-to-liquid (Figure 1). On the other hand, biomethanol refers to green methanol produced from bio-based sources through bioprocesses with the involvement of microorganisms and enzymes. As shown in Figure 1, e-methanol represents green methanol which is produced from CO2 capture as a byproduct of the electrolysis process to generate hydrogen. AlNouss et al. [3] developed the gasification of palm wastes for methanol production and investigated a techno-economic and environmental analysis. The focus of the study is CO2 capture by deploying CaO to maximize methanol production and profit. It is widely acknowledged that fuels derived from biomass resources can reduce CO2 emissions and partially replace fossil fuels. Regarding the economic aspect, the project life, methanol and dimethyl ether prices affected the economic feasibility of the biomass conversion process [4]. In consideration of the current energy consumption trends, efficient utilization of biomass resources has increased in both the industrial and academic sectors. Galusnyak et al. [5] examined a new membrane process design from secondary biomass for biomethanol production. As the results show, the utilization of exhausted olive pomace as a biomass source would enhance the detailed distribution of the global warming potential impact category.
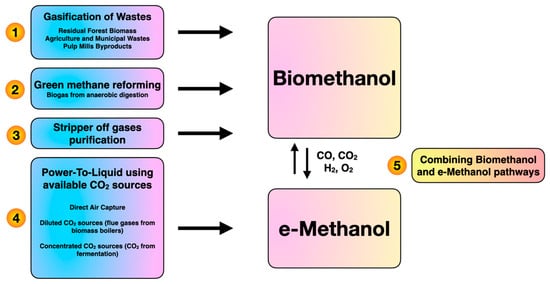
Figure 1. Main pathways for methanol production.
Commonly, methanol, one of the ‘mega’ industrial platform chemicals, is produced from different feedstocks such as natural gas or coal. From Figure 2, the current worldwide methanol production capacity is 198 Mt/year, which is mostly employed for producing formaldehyde and plastics. Methanol is mainly produced from syngas (H2 and CO) via steam reforming of natural gas as well as coal gasification. The coal-to-methanol process significantly increases the energy consumption and CO2 emission to co-produce syngas and hydrogen [6][7]. The naval industry is also another fast-growing sector to consume methanol. Methanol production is predicted to follow the increasing trend up to 2050 since green methanol is attracting great interest as a sustainable energy carrier [7][6]. Chen et al. [8] proposed a novel hybrid process of coal gasification and coking to produce methanol. They investigated the effect of the coke gasification reaction and carbon tax on the integrated process economy. According to a detailed techno-economic analysis, the internal rate of return for coal gasification is 22.5% with more economic benefits in comparison with the coal-to-methanol process.
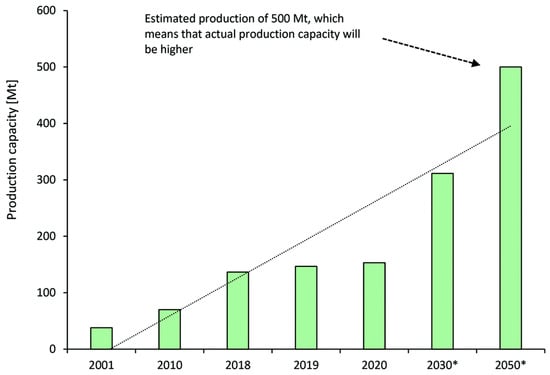
Figure 2. Worldwide production capacity and prediction for methanol in million metric tons (Mt). * Represents predicted production capacity.
The conventional synthesis of methanol is based on the reforming of fossil sources, which has raised environmental issues due to greenhouse gas emissions. Hence, it is important to generate methanol from biomass feedstocks and thermochemical processes. Kasmuri et al. [9] investigated the production of methanol from renewable sources via a thermochemical reaction. The dynamic control system was employed to maintain a high yield of methanol production in pyrolysis.
2. Potential of Methanol as a Renewable Energy Carrier
2.1. Industrial Applications of Methanol
Methanol has a variety of industrial applications in chemical industries, such as supplementing fuel oil, acting as an antifreeze agent in pipelines, and being used as fuel cells. Moreover, methanol is the simplest organic liquid hydrogen carrier and acts as an energy storage system for a variety of portable power applications. In terms of the transportation sector, Table 1 makes a comparison of biodiesel and gasoline (hydrocarbon fuel) in terms of energy density and average octane numbers. Methanol shows a wide range of downstream applications such as the substrate for producing biodiesel through transesterification and organic solvents. As shown in Figure 3 methanol can be suggested as a promising substrate in various sectors such as the generation of fuel, chemicals and light olefins.Table 1. Summary of energy density and octane number for different alcohols.
| Fuel | Energy Density (MJ/L) | Octane Rating |
|---|---|---|
| Methanol (CH3OH) | 16 | 98.65/108.7 |
2.2. Techno-Economic Analysis of Methanol Production
Table 2 shows a list of some reported thermochemical and biological processes for methanol production.Table 2. Some reported thermochemical and biological processes of methanol production.
| Approach | Concluding Remarks | Future Prospects | Reference |
|---|---|---|---|
Table 3. Literature survey of sustainable production of methanol from different biomass wastes.
| Technique | Plant Size | Feedstock | End Product | Production Cost | Reference |
|---|---|---|---|---|---|
| Production of bio-methanol as potential renewable energy | Methanol can be produced from additional reactions of decomposed biomass material. Improvement in the electrolysis process and renewable electricity favors methanol synthesis. | In gasification and methanol synthesis the separation of gas and solid needs to be considered to reduce the environmental impacts. | [10][14] | ||
| Ethanol (C2H5OH) | 20 | 99.5/108.6 | |||
| Propanol (C3H7OH) | 24 | 108/118 | |||
| 2 | |||||
| H6O) | 18.9 | - | |||
| C8H18 (2,2,4-Trimethylpentane) | 33 | 85–96/90–105 |
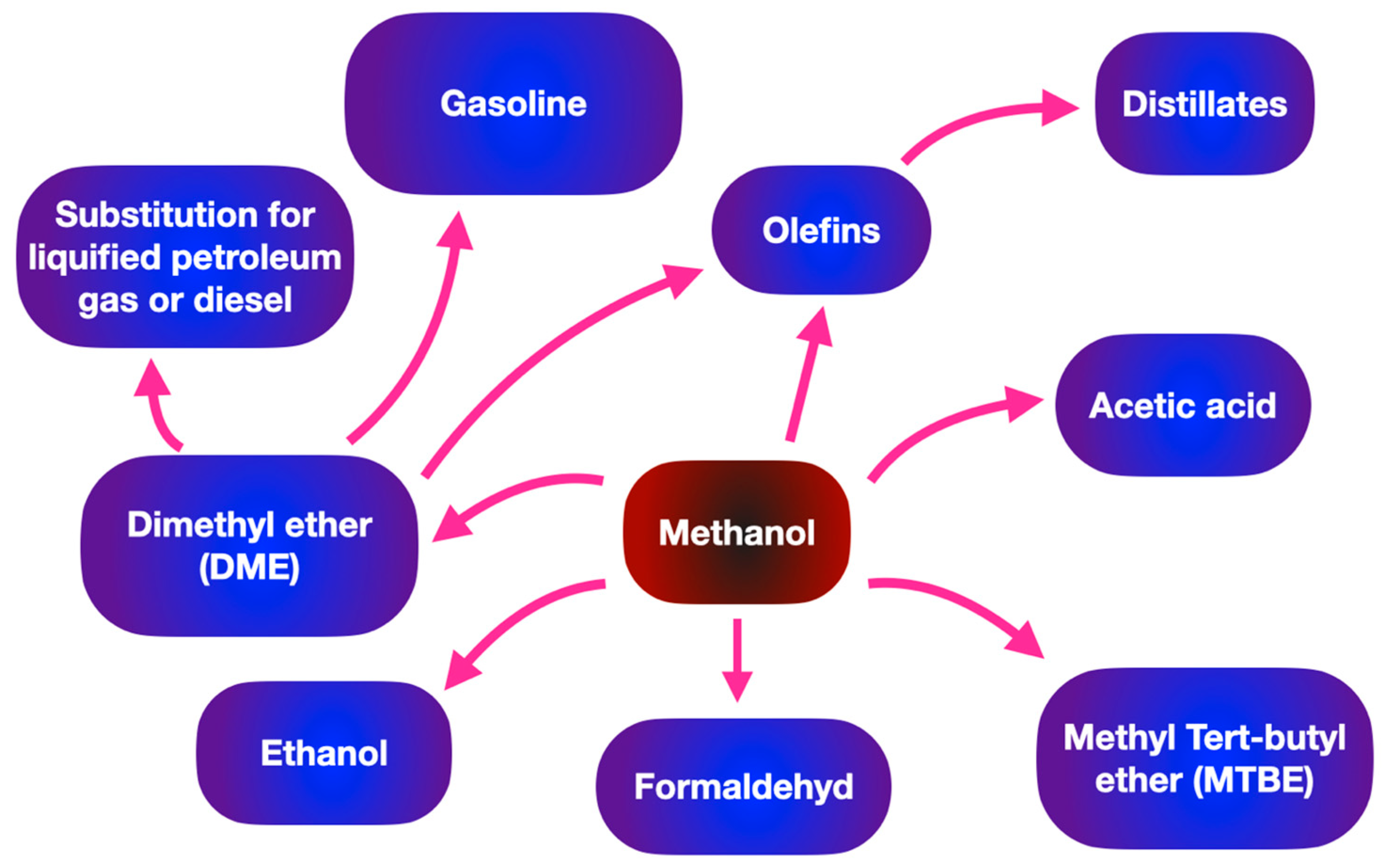
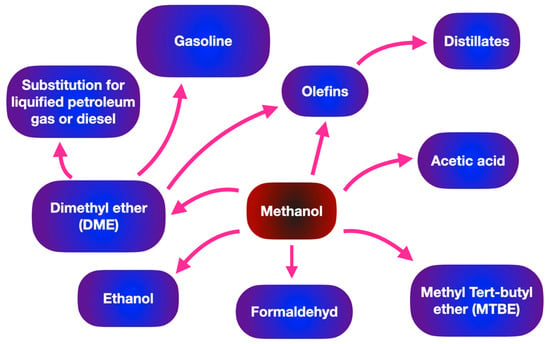
Figure 3. Wide range of applications of methanol in fuel and chemical industries.
| Methanol fuel production | Methanol production and techno-economic viability are influenced by feedstock characteristics, initial investment, and plant location. | Dimethyl ether alongside methanol has a huge potential to be a new generation of fuel source owing to its low calorific value, low density, and low viscosity. | [11][15] | |
| Methanol production from different renewable sources and thermo-economic analysis | The thermo-economic analysis considering different scenarios confirm that the best economic results are obtained with hydroelectric source. | Considering future methanol selling prices of 500 Є/ton, the economic performances can be further improved via the European financial incentives for biofuel production. | [12][16] | |
| Butanol (C4H10O) | 30 | 97/103 | ||
| Sustainable production and application of methanol | The main advantage of biomass-derived methanol is the eco-friendly aspects of methanol production as a clean fuel. | Biomethanol is a future bioproduct for value-added industries due to its diverse applications. | This study | Dimethyl ether (C |
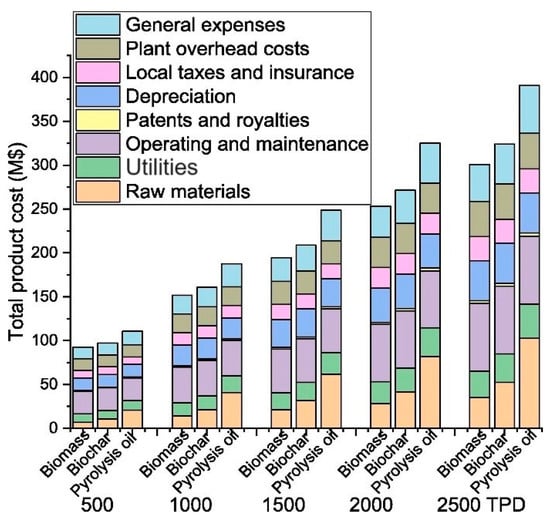
Figure 4. Effects of different feedstocks on the total product costs at various plant scales. TPD represents tons per day.
Table 4. Economic and environmental aspects of methanol production via biochemical and thermochemical processes.
| Process | Environmental Impact Analysis | Economic Evaluation | Reference | ||
|---|---|---|---|---|---|
| Biomass torrefaction coupled with gasification |
Processing rate of 66.4 tons/h |
Bagasse | Electricity and /or methanol production | ||
| Methanol production from wood biomass | Both production processes had a much lower CO2 emission compared to fossil fuel-based methanol production. | The rectisol-based acid gas removal unit used for removing sulfur and CO2 from syngas corresponds to 20% of the total investment costs. | [25 | The annual production cost was estimated at USD 140 M/y | ][29][16][20] |
| Sugarcane biorefineries with fossil fuel co-combustion |
Processing rate of 421,000 t/y | Lignocellulose | Methanol production of 82,700 t/y and electricity production of 3.5 GWH/y | 272.6 USD M/y | [ |
| Biomethanol production from palm wastes steam gasification | The reduction in CO2 using CaO was effective with a slight increase in the total cost of the plant. | The total capital cost is approximately USD 120 M. | [3] | 17 | ][21] |
| Tri-reforming of CH4 integrated with solid oxide fuel cell | Annual cost of USD 277,742 /y |
Methane | Methanol production | 5.4 USD M/y | [18][22] |
| Synthetic methanol production from H2 and CO2 |
243 MT/day H2 and 1978 Mt/day CO2 | CO2 and H2 feedstocks | Methanol production is 1190 Mt/day | The minimum fuel selling price of methanol was between USD 0.61/kg and USD 0.64/kg | [19][23] |
