The liver is the largest gland in the human body, mediating essential functions in homeostasis, metabolism, serum protein production, storage of glycogen, drug detoxification, immune system, and production and secretion of bile acids. The ultimate goal of liver tissue engineering (LTE) is to restore partial or total function of the liver during liver failure. A fully functional liver is the ultimate aim of LTE, and functional liver tissue can be used for drug testing. Moreover, LTE has the potential to develop an extracorporeal liver support (ECLS) system performing the essential functions of the liver to reduce mortality or to bridge a patient to a liver transplant. With recent advances in the field of regenerative medicine, tissue engineering holds high potential to progress as an alternative or supplement to LT. A major advance in the LTE area was published by Chhabra and colleagues, reporting the development of a vascularized liver model to understand liver regeneration.
1. Focus on Extracellular Matrix: Use of Biodegradable Biomaterials
Tissue engineering using scaffolds with biodegradable properties and structures is the most popular method and is capable of maintaining three-dimensional (3D) cell growth, tissue regeneration and overall function of the engineered liver tissue
[1][26]. Since the liver is one of the organs with the most vascularization, it is ideal to have a biomaterial compatible with supporting vascularization. The scaffold can be designed to gradually degrade, leaving behind only the regenerated liver tissue once the engineered liver tissue matures and becomes functional. The major biophysical factors critical for ideal cell transplantation during tissue regeneration are (i) porosity, which generally affects cell adhesion, proliferation, migration, and differentiation; (ii) stiffness, also affecting the hepatocellular phenotypic properties; and (iii) geometry, directly influencing the seeded cells
[2].
Commonly used biomaterials addressing the requirements for the hepatic cell types and ECM are mainly natural hydrogels and synthetic materials
[2]. Hydrogels like alginate, chitosan, and gelatin have advantageous effects for promoting and restoring cell growth and function
[3][8]. Marine polysaccharides and marine-derived chitosan show protective effects against liver damage while serving as scaffolds for tissue engineering
[4][27]. The ECM of the liver contains collagens, which are the most abundant component. Several studies carried out using modified collagen for liver tissue engineering showed positive outcomes with the expression of hepatic markers of cell spheroids
[1][26]. Hyaluronic acid, a commonly used matrix component that binds to CD44, is expressed by immature and mature hepatocytes. Hepatocytes that are formed as cell aggregates in hyaluronan scaffolds remained viable and proliferative active for more than 4 weeks
[5][28]. Matrigel contains a mixture of proteins from murine chondrosarcoma composed of laminin, collagen type IV, proteoglycan, and heparan sulfate
[6][29]. Several LTE model studies have used Matrigel as a scaffold for culturing hepatocytes and fostering stem cell differentiation. However, inconsistent mechanical properties, degradability, and lack of regenerative ability, along with potential xenogeneic and tumorigenic origin, are some of the shortcomings in the usage of these natural biomaterials, especially for supporting LTE for clinical applications
[7][1]. Synthetic materials containing biodegradable polymers like polylactic acid, polyanhydrides, poly-L-lactic acid, and polycarbonates have more superior properties and support regeneration, transplantation, and biodegradation
[8][30]. By modifying these synthetic biomaterials by incorporating proteins or bioactive domains, biocompatibility can be improved in these synthetic biomaterials
[7][1]. Decellularized ECM has the advantages of compatibility and degradability compared to other natural or synthetic media, which will be discussed in more detail later in this re
svie
archw.
2. Knowledge from Studies on Post-Hepatectomy Liver Failures
Understanding the impact of the cytokine and growth factor signaling pathways involved in post-hepatectomy can be beneficial for optimizing LTE. Along with optimizing biomaterial scaffold, a proper balance of these factors can be recreated in in vitro conditions to improve the regenerative potential of the liver. Hoffmann et al., reported an elaborative study where modulation of several potential cytokines and growth factors were analyzed from 3353 articles, including around 1000 animal studies and a double number of human studies
[9][10][22,31]. In this article, a number of potential predictive biomarkers and their implications for liver diseases were identified and discussed
[10][31]. A list of cytokines/growth factors relevant to LTE are listed (
Table 1)
[11][32]. Incorporation of high-throughput screening studies using ex vivo regenerative liver models can be useful to further validate the biomarkers identified.
Table 1.
Potential biomarkers in liver diseases relevant to liver tissue engineering.
3. Whole-Organ Bioengineering Approach for Liver Tissue Engineering
Whole-organ bioengineering involves gentle decellularization using detergent solutions to retain the dimensional ECM with preserved architecture, including vasculature
[12][33]. The native framework after decellularization can ultimately be used for successful organ transplants. This strategy is highly beneficial to utilize organs destined for human transplantation but discarded because of diverse reasons
[13][34].
Whole-liver bioengineering has been in focus among LTE approaches
[14][35]. Diffusion and perfusion techniques have been tested for liver acellularization
[15][36]. Baptista and colleagues reported that the decellularization of the liver matrix showed favorable conditions for the survival of human fetal liver cells and human umbilical vein endothelial cells
[16][37]. However, one of the major challenges in whole-liver bioengineering is repopulating the scaffold using appropriate cell type and source. Induced pluripotent stem cells were tested to produce functional hepatocytes in mice
[17][38]. Further research on growing diverse primary liver cells to repopulate liver scaffolds, including hepatocytes, HSCs, and LSECs, is urgently needed. Once optimized, this powerful technique can preserve liver architecture, liver-specific ECM, and vasculature for cell survival and function with a multitude of applications, as shown in
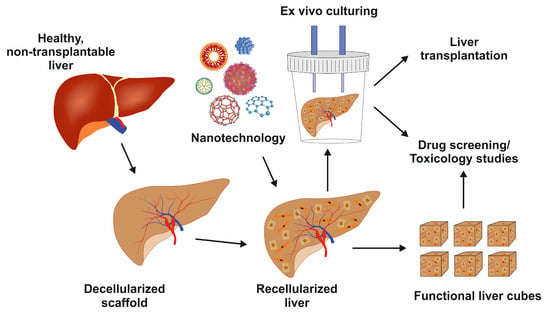
Figure 14.
Figure 14. Image showing the decellularized liver scaffold, recellularization with nanotechnology support, and its potential uses for liver transplantation, drug development, or toxicology studies.
4. Recellularization in Liver Tissue Engineering
Repopulating the scaffold, either biomaterial-derived or decellularized, is complex as it involves parenchymal spaces, macrovascular lining, and biliary tree
[18][39]. Parenchymal repopulation can be carried out using variable cell sources cultured in adequate quantities in an ideal environment, including hepatoblastoma cells like HepG2 cells. Primary hepatocytes were also tested in animal models, showing high functionality
[19][40]. They have the advantage that they have, in clinical application, a much lower tumorigenic potential than the immortalized cell line HepG2
[20][41]. It has been further reported that rat primary hepatocytes stay viable for 14 days in decellularized Wistar rat livers with the capacity to express typical lineage markers
[18][20][39,41].
Primary hepatocytes serve as a potential alternative to mesenchymal, embryonic, or induced pluripotent stem cell-derived hepatic cells
[21][42]. The potential advantages of these cells include availability, a more mature phenotype, and a consistent cell source. Under physiological conditions, hepatocytes remain quiescent and proceed to the G
1 phase upon liver damage, which leads to their proliferation. Understanding hepatocyte proliferation is of utmost importance as it constitutes the majority of the functional liver. Elchaninov et al. demonstrated in rat models that there is delayed hepatocyte proliferation following subtotal hepatectomy
[22][43]. Additionally, there is a delicate balance between pro- and anti-mitotic paracrine factors that determine their proliferation status
[22][43]. The induction of Sox9 transcription factor, a major indicator for hepatocyte proliferation, and two genes encoding tumor necrosis factor-like cytokine TWEAK (TNFSF12) and its receptor Fn14 (TNFRSF12A) in the management of hepatocyte and cholangiocyte proliferation were reported
[23][44]. However, with optimized protocols, stem cell-derived hepatocytes can be mass-produced with customized functionalities. Human mesenchymal stem cells (hMSCs) can be modified to support liver functions. During their culturing in in vivo conditions, they retained the expression of hepatic markers compared to their 2D cultures
[13][34]. Human-induced pluripotent stem cells (hiPSCs) can be reprogrammed into several cell types and differentiate effectively into hepatocyte-like cells
[24][45]. One major bottleneck in using iPSC-derived hepatocytes is the time taken for the entire culturing and differentiation to ultimately establish functional hepatocytes
[24][45]. Recently, the group of Kamishibahara and colleagues reported optimized conditions using a Lamininin-511 (LN) direct coating and specific factors to differentiate hiPSCs into hepatocytes, mesodermal cells, endothelial cells (EC), and mesenchymal cells
[25][46]. Liver organoids derived from these differentiated cells showed consistent hepatic functions
[25][46]. Kajiwara and colleagues demonstrated that the variations in hepatic differentiation were mainly determined by the donor differences rather than the derivation methods
[26][47]. Hence, autologous sourcing of hiPSC-derived liver cells can have an advantage. There are successful studies using differentiated hepatocytes from iPSC expressing hepatocyte markers and functioning in decellularized rat liver scaffolds
[27][48]. Cell sourcing and culturing strategies for vascular tracts and bile ducts is another major area of LTE for which advanced studies to develop efficient strategies are ongoing
[24][45]. Repopulating the decellularized liver may be a better approach with scaffolds in place after decellularization.
The long-term efficiency of using these cell sources or co-cultures for repopulating biomaterial/decellularized scaffolds is yet to be determined. In particular, the ideal culture conditions are shown to be more in a complex or 3D format, mimicking the in vivo environment. Some of the studies where functional cells were successfully cultured will be discussed in the next session.
5. Liver Cell Culture Methods
There are several ways reported to grow functionally differentiated liver cells. Yang et al. recently extensively reviewed various methods for biomedical applications. Some of the reported bottom-up tissue culture methods include micromodel co-culture, spherical aggregate culture, and cell sheet culture methods
[24][45]. Nanofiber scaffold,3D bioprinting, and decellularization-cellularization methods are additional top-down tissue engineering methods
[24][45].
However, with reduced complications in requirements for the vascular cells or other ECM requirements, decellularized scaffolds can be advantageous. Park et al. used iPSC-derived porcine hepatocytes to populate decellularized porcine liver scaffolds
[28][49]. The recellularized liver cells showed hepatic markers and functionality after culturing using a continuous perfusion system. Further, these recellularized scaffolds were successfully transplanted into rats, highlighting the potential of this approach
[28][49]. A high-shear stress oscillation-decellularization method was employed by Mazza et al. to generate human acellular liver tissue cubes (ALTCs)
[29][50]. These ALTCs were later seeded with functional liver cells derived from human parenchymal and non-parenchymal liver cell lines and human umbilical vein endothelial cells (HUVECs)
[29][50].