Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Maurice Millet and Version 3 by Lindsay Dong.
Fog water have been rapidly increasing due to its negative impacts on different environmental processes. However, fog water harvesting has become beneficial in various countries to overcome water scarcity. Accurate fog forecasting remains a challenging issue due to its spatio-temporal variability and uncertainties despite the development and efforts made to understand its chemistry and microphysics. The literature proved that the decrease in fog frequency over time in most countries is mainly attributed to the improvement in air quality or the change in regional climatic conditions.
- fog impacts
- fog lifecyle
- fog
1. Introduction
Atmospheric pollution, characterized by measurements of biological, physical, or chemical contaminants, has become an international policy problem having harmful consequences for human health and ecosystems [1][2][1,2]. Accordingly, fog droplets are composed of a mixture of organic and inorganic compounds resulting from scavenging hygroscopic particles and water-soluble trace gases. Fog droplets are formed on aerosol particles in a supersaturated atmosphere. However, the pollution loadings of these particles are higher than those in clouds and precipitation [3]. It is the result of the synergic effect of weather factors (relative humidity (RH), wind direction (WD), wind velocity (WS), temperature (T), pressure (P), etc.) and pollution (presence of aerosols) [4]. Its chemical composition is an important tool for the complementary analysis and identification of air and long-range transport (LRT) pollutants because fog is formed in a shallow boundary layer which is conducive to fog formation and has the ability to trap local and regional pollutants. According to the American Meteorological Society (AMS), fog comprises a large number of small water droplets in the liquid form or ice crystals suspended in the atmosphere reducing the visibility below 1 km (0.62 miles) in the surrounding area [5]. Surface visibility is critical for aviation, transportation, and land safety, causing significant human and financial losses, and is responsible for serious air, land, and water transportation [6][7][8][6,7,8]. Modern aircraft have no difficulties with fogs of 1 km, but some restrictions can be imposed when the visibility degrades to lower than that. Visibility of less than 500 m is classified as fog [9]. The reduction in visibility depends on the resulting distribution of fog droplets and on the concentration of cloud condensation nuclei (CCN). The densest and thickest fog mainly occurs in urban or industrialized areas in the presence of a high number of polluted particles in the air acting as CCN for water droplets [5][10][5,10]. Fog is of great meteorological significance due to its strong relation to humidity factors and its capability of reducing the temperature amplitude and local character of formation. Its formation, existence, and dissipation are strongly influenced by numerous factors including local orographic conditions (changes in atmospheric conditions), atmospheric circulations (heat distribution by large-scale air masses), and the actual synoptic situation (pressure pattern, fronts, wind direction, wind speed, etc.) [10]. The presence of natural fog affects many environmental components including global and local climate, air quality, water quality, air–surface interactions, the thermal and radiative budget of the atmosphere, etc. [11][12][11,12]. It has severe impacts on social life leading to an increase in the number of injuries due to the reduced visibility whether in water, air, or land transportation [5][12][5,12]. Fog has also direct and indirect adverse impacts on human beings (primary and secondary) [13]. However, it has a beneficial effect on decreasing the concentration of different air pollutants, cleaning the atmosphere through the wet deposition phenomenon, and agricultural and water supply activities [14][15][14,15]. Therefore, monitoring these climatic events in the different environmental matrices is crucial to better understand the consequences of their presence in the environment and to meet the criteria for defining good air quality.
Fog droplets acquire their chemical composition by mechanisms similar to those of cloud water droplets [16][20]. The solute concentrations in fog water (organic acids, heavy metals, ions, etc.) are usually up to 100 times higher than those observed in precipitation due to the longer residence time in the atmosphere and their smaller droplet size. The longer residence time makes possible the higher accumulation of the products of the liquid-phase processes. The major composition of fog water is the result of the interaction of sulfur dioxide (SO2), nitrogen oxide (NOx), carbon dioxide (CO2), hydrogen chloride (HCl), and ammonia (NH3) with water in an oxidative atmosphere (oxygen (O2), ozone (O3), sunlight, etc.) and in the presence of trace metals that may act as redox catalysts (iron (Fe), copper (Cu), manganese (Mn), and organic materials including dust, soot, and hydrocarbons (HCs)) [17][21]. Fogs act as a micro-reactor for chemical and photochemical reactions with atmospheric oxidants such as singlet oxygen, hydroxyl radical, nitrate radical, etc. They are important media for aqueous-phase reactions where inorganic gases such as SO2, NOx, and dissolved volatile organic compounds (VOCs) are oxidized.
2. Fog Types
Several points of view have been widely used in fog classification. It might be based on thermodynamic properties (mixed-phase fog), physical (freezing and ice fogs) and dynamical processes (turbulence and mixing fogs), the chemical composition of particles (dry fog), weather features (frontal fog), and the physiographic character of the surface (valley fog). Another point of view suggests that fog might be divided into three categories: liquid fog, mixed-phase fog, and ice fog. The latter forms when the temperature falls below −10 °C, liquid fog forms when the temperature is higher than −10 °C, and the mixed-phase fog forms between −10 °C and −30 °C [18][24]. However, fog might also form under special conditions. For instance, ice fog can occur at a temperature of −20 °C in the case of excessive vapor being absorbed by ice nuclei under steady-state conditions in the absence of mixing processes [11]. Willett proposes a fog classification based on favorable synoptic conditions. He sub-categorizes them into numerous types that might be formed in the atmosphere [19][25]. They include advection fog, valley fog, upslope fog, freezing fog, ice fog, steam fog, precipitation fog, and radiation fog [11][20][21][22][23][24][11,26,27,28,29,30]. Each type of fog is defined by a special physical process responsible for its formation [11]. Advection fog results in locations where warm air passes over cooler ocean water. As this process occurs, the temperature drops to the dew point, and water vapor condenses in the warm air, producing an RH of 100% and leading to the formation of fog. It mainly occurs in windy conditions such as on the Pacific coast of the US and San Francisco where the ocean is significantly cooler than the surrounding land. Typical fogs extend up to a few hundred meters in height [25][31]. Valley fog is formed during winter in mountain valleys where the dense air is trapped in the valley. In this area, the dense air settles down the valley and condenses to form fog. It is essentially due to a temperature inversion along with warmer air flowing above the valley. It may last for a few days during winter in calm conditions. Upslope fog occurs when the air flow rises up the terrain and cools it adiabatically to its saturation temperature allowing water vapor to condense to form fog. When it is seen from below, it is viewed as stratus clouds; as one goes up into the cloud, it is viewed as fog. This type is also known as the orographic fog. Freezing fog is formed when water droplets in the air mass become supercooled, and solid surfaces are frozen. As the fog intensity increases, the ground, trees, and other objects are glazed by a layer of rime or frost. Freezing foggy events occur at temperatures below 0 °C. Ice fog develops in Polar or Arctic regions where air temperature is below freezing. It is usually observed at high altitudes, in calm and clear weather, and in extremely cold air (<−29 °C). Ice fog is composed of ice crystals suspended in the air instead of supercooled water droplets. It results when water vapor is released into the atmosphere and is then condensed to form droplets that are rapidly frozen into ice particles. Steam fog is somehow the reverse of the advection fog. It occurs when cold air passes over relatively warm water. The air is cooled and moistened, causing the dew point to increase, causing condensation of water vapor leading to the formation of fog. It is a common phenomenon occurring during early winter and autumn, in middle latitudes, near rivers and lakes where water is still warm [26][27][28][32,33,34]. Precipitation fog is associated with weather fronts, especially warm frontal boundaries. It is formed when warm rain falls through cold and almost saturated air. When the precipitation falls down into colder air, the quantity of water vapor in the atmosphere is increased through evaporation, causing the dew point to rise and the cool air to become saturated to form fog. It is also known as frontal fog [25][29][30][31][32][31,35,36,37,38]. Radiation fog is known as ground or continental fog that does not reach any of the clouds overhead. It usually occurs at night under stable conditions (clear sky and calm wind) and dissipates in the morning as the ground warms by increasing the heating rate from thermal radiation. This type is common in continental climates during winter under anticyclonic conditions (high pressure). Radiation fog is produced when the heat absorbed by the surface is radiated into the air, cooling the ground and causing a temperature inversion. As the surface cools, a layer of moist air is created near the ground and reaches its dew point. At this point, condensation occurs, resulting in the formation of fog [33][39]. The depth of the radiation fog increases as long as sufficient moist air is available. Typical ground fogs reach 100 to 200 m in height. Radiation fog is a mixture of liquid droplets, gaseous species, wet aerosols, and dry PM, resulting from complex interactions among these phases, contributing to the enrichment of fog droplets with inorganic and organic contaminations. Briefly, two basic concepts lead to fog formation: either air temperature reaches the dew point, forming advection, upslope, and radiation fog, or sufficient vapor is added to the air, forming frontal and evaporation fog [34][40].3. Fog Forecasting
Fog is an important meteorological phenomenon that should be predicted accurately due to its strong influence on the economy and personal safety. Poor forecasting leads to a greater disruption to surface, sea, and air transport with subsequent increased risk to the economy and personal and social life. Fog is influenced by numerous factors, covering multiple temporal and spatial scales [11]. Fog formation is correlated with the presence of meteoroidal conditions such as low temperature, wind speed, wind direction, and very high relative humidity. In fact, fog does not always occur in windless and calm conditions. A historical dense foggy event was reported by Scott in 1896, stating that fogs with strong winds accompanied by heavy rain occurred in the British Isles. Fog-related events associated with strong winds are estimated to be about 135 in 15 years [35][36][41,42]. In 1892, fog formation was found to be related to the role of aerosols. Mensbrugghe states that “aqueous vapor condenses in the air only in the presence of solid particles around which the invisible vapor becomes a liquid” [37][43]. Additionally, Willett emphasizes the importance of CCN in fog formation. He reports that dust particles, hygroscopic particles, and those having an electric charge or ions facilitate fog formation [19][25]. The presence of hydrophilic particles is an important key that facilitates the condensation of water vapor into fog droplets. The pollution does lead to fog formation, and the heterogeneous nucleation of pollution condensation nuclei leads to fog droplet formation. The increasing quantity in polluted atmospheres decreases the surface tension, causing the pollution particles to be activated at lower relative humidity and resulting in a denser and thicker fog [38][44]. All these factors lead to the accumulation of pollutants in a stable and strong inversion boundary layer which is responsible for fog maintenance. Thus, air saturation with water vapor and favorable meteorological conditions are two driving parameters of fog formation. Fog appearance and dissipation are still not very clear since they are directly related to turbulence, microphysical and radiative processes, thermodynamics, and surface conditions. The reason behind that could be that fog is sensitive to the complex balance mechanism among all the processes. The initial conditions of turbulence and humidity are critical for the prediction of fog events. Some researchers suggest that fog is formed due to a turbulent mixing between nearly saturated eddies with slightly different temperatures when the colder air mixes and cools the hotter moister air reaching the saturation. Other researchers suggest that a virtual cessation of turbulence is necessary before fog formation. In this mechanism, it is assumed that the high levels of turbulence cause saturation to occur at the surface in the form of dew, preventing the coalescence of fog droplets and fog formation. Once formed, its further maturation or dissipation depends on the evolution of its physical processes and the environmental conditions that govern the removal and production of liquid water. Overall fog forecasting remains difficult and challenging since it depends on a large number of physical and chemical processes along with non-linear interactions. Factors that may be involved are many: atmospheric stability, radiation balance, moisture availability, turbulence, advection, topography, and microphysics [39][45]4. Fog Frequency
The United States National Oceanic and Atmospheric Administration (US NOAA) runs the Global Daily database from over 8000 stations worldwide to make a useful comparison regarding the average annual fog frequency. A twenty-year period, between 1991 and 2010, is investigated to obtain precise meteorological data. The annual number of foggy days and annual cycle of fog widely vary according to local conditions and weather factors. The high occurrence of fog water usually occurs where water vapor is in excess such as in locations near the ocean, river, lake, sea, and other humid sites as well as where favorable conditions are present (i.e., cooling). Other factors including local conditions (altitude, type of land), mesoscale (distance to the coast, exposure to advection air masses), and synoptic scale (cyclonic scale) affect the duration and frequency of fog. The highest fog frequency has been observed in Washington/US (NDF = 311), Śnieżka/Poland (298), and Harz/Germany (284). High fog occurrence has also been detected in equatorial and subequatorial zones such as in Iquitos/Peru (102), due to the extremely high humidity and nocturnal radiative cooling. Fog occurrence is found to be high in montane tropical atmospheres such as in Quito/Ecuador (208). Further, in tropical and subtropical zones next to the coast, fog frequency is also high such as in Chile (189), where the region is influenced by cold oceanic air masses. In polar zones, the occurrence of foggy events is also high such as in Nuuk/Greenland (81) and Marambio/Antarctic (138), where advection fog is dominant. However, fog frequency all over the world has shown a significant decrease. Statistical results show that the strongest relation to fog occurrence is air pollution. In the case air pollution becomes less severe, environmental factors such as wind speed, urban heat island (UHI), relative humidity, and inversion layer become decisive in controlling its occurrence and duration [40][124]. Literature studies reported a decrease in the majority of fog stations worldwide. Williams et al. reported that the change in regional climatic conditions such as the ongoing intensification effect of UHI or atmospheric circulation leads to an increase in air temperatures contributing to a decrease in the fog frequency in southern California [41][42][89,125]. Aerosols are found to have a direct relation with fog evolution [43][126].5. Fog Nucleation and Activation
5.1. Fog Nucleation
The formation of fog water requires relatively high humidity conditions ranging from undersaturated to slightly supersaturated conditions [44][140]. The presence of atmospheric aerosols is a key factor for droplet formation, where they can grow in size more easily in a saturated atmosphere [38][44]. The process of forming the droplet nuclei, known as the nucleation scavenging process, is of great significance for cloud, particle, and fog formation [45][141]. The presence of hydrophilic inorganic species including sulfate, nitrate, and ammonium (SNA) and soluble elements including magnesium and calcium plays a vital role in fog formation by acting as CCN. The presence of water-soluble organic carbon (WSOC) has been pointed out to modify the hygroscopicity of aerosol particles and enhance their tendency to act as CCN [46][142]. The availability of trace metals like copper, manganese, and iron also plays a significant role in aerosol–fog interactions by acting as catalysts for aqueous-phase reactions. Numerous types of AA particles are capable of acting as CCN. Some of the AAs are generated from natural sources (sea spray, volcanic debris, biogenic aerosol, etc.), while others are derived from human-made activities (industrial emissions, agricultural activities, biomass burning, fossil fuel combustion, etc.) [47][48][23,147]. In the presence of small amounts of water supersaturation, AAs tend to grow spontaneously to form fog droplets. Particles containing water-soluble compounds are more desirable to act as CCN over those containing largely insoluble compounds [49][148]. Particles with diameters ranging from 0.001 to 0.2 µm play a significant role in fog/cloud and precipitation microphysics. In fogs, aerosols are activated and grow into droplets whenever their size is greater than 0.1 µm and smaller than 1 µm. This means that the accumulation-mode particles are mainly responsible for fog formation. Particles with diameters greater than 1 µm may grow but without being activated [49][50][51][148,149,150]. In the case the particle concentration is high and/or the supersaturation level is low, the minimum size of the particle required for activation is 0.5 µm [51][150]. The accumulation-mode particles are formed through the coagulation of smaller particles that belong to the Aitken nuclei (<0.1 µm) or from the condensation of vapors into existing particles, forcing them to grow [52][151]. They are characterized by their long residence time and high concentrations compared to other modes. The accumulation-mode particles account for a substantial fraction of the total aerosol mass and have the greatest surface area. This makes them of high importance to atmospheric heterogeneous chemistry. Such particles are released through the incomplete combustion of coal, oil, wood, gasoline, etc.5.2. Fog Activation
For a complete understanding of the activation process, the size distribution and chemical composition of AAs must be taken into consideration. The size of AAs is highly connected with water vapor supersaturation in fogs which is a key for the activation process. Particles are divided into two categories: activated and non-activated particles. In the case the critical supersaturation level (SScr) is lower than the actual supersaturation, particles are activated. In the case the SScr is higher than the actual supersaturation, particles grow to their equilibrium diameters by capturing water but remain inactivated [53][54][144,159]. Thus, the ability of CCN to be activated into droplets is determined by the physical and chemical properties of AAs [51][55][150,160]. The spontaneous growth of CCN into fog droplets under supersaturated water vapor conditions is described by the classic theory of Kohler. The rate of droplet growth depends on the initial size of aerosols and their solubility [56][161]. CCN activation depends on the interrelation between the Raoult effect—known as the water activity—and the Kelvin effect. In the Raoult effect, the potential of CCN activation increases with decreasing water activity or increasing solute concentration. In the Kelvin effect, the potential of CCN activation decreases with the decreasing size of the water droplet or increasing surface tension. Through the Raoult effect, certain aerosol particles absorb water vapor at a relative humidity below 100%, and then they grow in size. In this way, they reach sufficient diameters for the Kelvin effect to occur leading to the creation of droplets, absorbing water vapor at their disposal. The transition from the Raoult effect to the Kelvin effect is the activation process of CCN [54][57][159,162]. Therefore, aerosols are considered to be “activated” once these droplets reach a certain size, where they are more easily grown within a saturated environment [56][161].5.3. Effects of CCN
The radiative effects of aerosols on fog may be classified as direct, indirect, and semi-direct [58][163]. First, aerosols may scatter and absorb solar radiation (short and long waves). Second, aerosol particles may scatter, absorb, and emit thermal radiation. Third, aerosol particles may act as CCN. The first two mechanisms are the direct effects, while the last one is the indirect effect. The semi-direct effect is a consequence of the direct effect of absorbing aerosols [59][60][61][62][164,165,166,167]. Direct radiative forcing of aerosols may either cool (nitrates, sulfates, etc.) or warm the atmosphere (black carbon (BC)), depending on the proportion of the scattered light to the absorbed light. The scattering aerosols have a cooling effect on the atmosphere, whereas the absorbing aerosols have a heating effect on the atmosphere. Absorbing CCN is also known to influence fog formation because when air temperature increases, the relative humidity is reduced, prohibiting the appearance of fog or shortening its life in the case it is formed through enhancing droplet evaporation [63][168]. This is one of the main reasons for declining fog frequency along with the effects of urban heat intensification [42][64][65][66][125,169,170,171]. Both direct and semi-direct effects of aerosols have been studied by Bott using numerical simulations. The latter shows that urban aerosols containing particularly soot absorb solar radiation and thus increase the cooling rate of the surface and accelerate fog formation. This is the direct effect. The same aerosol that absorbs more solar radiation leads to fog dissipation. This is the semi-direct effect [58][163].5.4. Droplet Size Dependence
The chemical composition of fog varies according to the droplet size mainly due to two reasons. The first reason is the inhomogeneous chemical composition of the CCN, while the second reason is the differences in the solubility rates of the gas uptake by small and large fog droplets. Smaller fog droplets are much more concentrated and grow faster than larger droplets as long as there is enough water vapor for condensation. The possible explanations for the enrichment of major inorganic solutes and organic carbon in smaller fog droplets include the higher dissolution rate of CCN in smaller amounts of water, differences in condensational growth, and the higher surface/volume ratio of the small droplets promoting greater surface area for gas/liquid transport and consequently more chemical reactions. The distribution of the chemical components across the aerosol size distribution depends on the chemical composition of the CCN on which the fog droplets form. The smallest activated droplets are formed on the smallest CCN, whereas the largest droplets are formed on the largest CCN. Thus, species contained in small accumulation-mode particles (e.g., SNA) are enriched in smaller fog droplets, and species originally found in the coarse mode such as calcium, magnesium, chloride, and sodium are enriched in larger fog droplets. However, large droplets are unnecessary to be more diluted than the smaller droplets. Through numerical simulations, Pandis et al. stated that droplets whose diameter is 20 μm have a bigger solute concentration than 10 μm droplets by a factor of 3.6 [67][174]. Thus, fog chemistry varies from one case to another with droplet size including the rate of condensation on CCN, rate of dilution, rate of soluble gas uptake, and rate of chemical reactions (e.g., S(IV) oxidation) [68][16].6. Fog Impacts
6.1. Air Quality
The high fog frequency in a particular region affects the air quality of that region. The most popular effect of the fog–aerosol interaction is commonly known as haze or smog (a combination of smoke and fog) [69][70][178,179]. There are mainly two forms of smog: the classical smog (London type) [71][180] and the photo-chemical smog (Los Angeles type) [72][181]. The latter is caused by the interaction of CO, O3, VOCs, and NOx with solar radiation and occurs near mid-day, especially during the summer season [73][182]. The classical smog is caused by the interaction of SO2 with PM and takes place in winter and autumn near the ground at temperatures around 0 °C in windless conditions. Once the haze fog contains the atmospheric pollutants, air quality decreases. The trapped NOx and HCs near the ground surface are converted into harmful O3. The greenhouse gases (GHGs) highly spread in the air are also trapped within the stable layer of the inversion zone. All these trapped pollutants in the inversion layer remain suspended and will have an adverse impact on the ecosystem and climate change.6.2. Human Health
Depending on the chemical and physical nature and composition of fog droplets, fog water has direct and indirect adverse effects on human health (skin and eye damage, respiratory and radiation diseases, secondary health effects, etc.) [74][75][76][186,187,188]. Exposure to fine aerosol particles, especially acidic species, nitric acid sulfur dioxide, sulfur oxide, and microbes, increases the morbidity and mortality of diseases in the respiratory system, cardiopulmonary system, throat irritation, cardiovascular system, muscular system, and lung cancer [74][77][186,189]. Exposure to sulfuric dioxide tends to affect the respiratory tract, leading to aggravation in asthmatics. Exposure to nitrogen dioxide demonstrates a slight unfavorable impact on the respiratory system at ambient concentrations. The inhalation of fog with high sulfuric acid concentrations has no clear influence on pulmonary activity, only a slight impact on the respiratory system [78][79][190,191]. The relation between asthma patients and air pollution or meteorological factors has been further investigated over a period of two years on 102 adults (44 patients are non-atopic while the rest are atopic). The results show that hospital visits increase on foggy days compared to fog-free days, especially on days with lower pH and low levels of gaseous air pollutants. An increase in hospital visits is observed when the concentrations of NO2 and NO are low in the case of non-atopic patients. Meanwhile, hospital visits of atopic patients increase with decreasing NO2 and SO2 concentrations. The reason might be possibly due to the scavenging of these pollutants by fog which could increase the acidity of fog water. Tanaka et al. state that adverse bronchial epithelium problems might be caused by several possible mechanisms associated with H+, O3, H2SO4, and HNO3 [74][186]. Thus, the fact that airway resistance caused by acidic pollutants increases might be due to the reduced absorption capacity of the hydrogen ions in the airway mucus [80][192]. The neutralization of naturally occurring acid fog with ammonia may reduce the impact of the inhaled acid aerosol during foggy days. Ammonia might neutralize about one-quarter of inhalable acid in healthy volunteers [81][193]. Concerning the meteorological conditions, the high ozone concentrations and the low day-to-day temperature differences lead to an increase in hospital visits for non-atopic patients. In both cases, high water vapor pressure favors the increase in hospital visits [82][194].6.3. Transportation and Economy
Fog affects a wide range of human activities. It may cause high costs, inconvenience, and even death [83][198]. It reduces visibility which acts as a barrier for driving, sailing, or even flying [84][199]. The total financial and human loss for fog-related transport accidents (sea, air, and land) is approximately the same as that of tornadoes, hurricanes, and winter storms in some cases [11][85][11,56]. Adverse visibility and ceiling conditions lead to 35% of weather-related accidents in civil aviation and cause, on average, 168 mortal casualties per year. In fog-prone regions, fog is cited up to 10% of the time as the principal source of accidents, especially in multiple-vehicle crashes. In the case of dense foggy events, airports refuse to accept planes and cancel take-offs due to security reasons such as the cases reported in Canada and the US [6][86][6,200]. This may cost the airlines between USD 5,000 and USD 25,000 for any delayed or canceled flight. Concerning sea transportation, many shipping operations are either stopped or slowed-down in the case where the visibility is lower than 0.5 km, and the economic losses typically range between USD 10,000 and USD 25,000/day/ship and cost millions of dollars for moderately active ports [11]. The estimation of the economic losses in 2006 associated with dense fog events in the pre-Christmas period was at least GBP 25 million at seven British airports. Approximately 50 people die yearly in Canada due to vehicle accidents in which fog is the main cause. Fog-related accidents resulted in 13,720 deaths between 1995 and 2004 in the US. The presence of fog led to 1122 fatal air accidents and killed around 229 people in the US between 1982 and 2013. In addition, the Aviation Safety Network reported that six planes have crashed in Iran due to poor visibility conditions, and 353 persons have been killed since 1988 [87][201]. Overall, a huge number of deaths is reported worldwide in fog-related ship collisions, vehicle crashes, and aviation [84][199].6.4. Benefits
Despite the severe negative impacts of fog around the world, it proves its beneficial impacts in terms of water applications, agricultural applications, and ecosystems. Fresh water scarcity has become a major problem facing humanity and is expected to further intensify due to the rapid increase in population density and climate change. Fog harvesting started between 1901 and 1904 in South Africa, and continuous progress has been achieved regarding this issue. The number of its research interests addressing technical aspects, policies, community development, economics, and impacts has increased, in addition to the increase in the operational fog water collection systems over time [88][89][90][91][92][207,208,209,210,211]. The number of publications on fog water collection revealed a growing interest and has increased from 4 (between 1981 and 1990) to 223 (between 2011 and 2020), most of them focusing on the experimental or technical part of fog collection [91][210]. An efficient option to overcome this issue is fog water harvesting using either the standard fog collector (SFC) or the large fog collector (LFC) using mesh nets (Rachel nets). The mesh materials can be nylon, polyethylene, and polypropylene (Shade cloth) which are able to capture different quantities of water from fog [93][212]. The size of the SFC, which was developed by Schemenauer and Cereceda, is 1 m2, while that of the LFC varies between 40 and 48 m2, and the ratio of width to height must be between 2.5 and 3 [94][213]. The number of fog collectors depends on many factors such as fog thickness, duration, and frequency, along with the water demand and financial capacity. The collectors are placed perpendicular to the prevailing wind [95][214]. The cost of the LFC is regulated by the price of mesh varying between USD 25 and 50 per 1 m2. Fog collection starts when fog droplets come into contact with the mesh net standing perpendicular to the fog-carrying winds where they are impacted. As fog droplets increase in size by coagulation, they find their way to the collection reservoir by gravity.7. Fog Collectors
Fog water collection has a long history. So far, several techniques and collectors have been developed and applied for the collection of fog samples including active and passive fog collectors. Passive collectors solve the problem of unavailable main power at the site. However, they might be contaminated by an unknown fraction of drizzle, conventional precipitation, and horizontal wind-driven rain. Many experiments employ passive fog collectors in which wind is the principal factor that drives fog droplets to the sampler where they are collected via their impaction on strings. The deposition plate is the simplest passive fog sampler. It includes a horizontal plate on which fog droplets are settled. The next fog collector is the string screen sampler. Fog droplets are collected through their impaction on a string screen. The droplets adhere to the string, move down along the string, and are collected in trays [96][232]. In active fog systems, the air flow containing water droplets is forced by mechanical means using either forced flow through rotating motors or fans and pumps to achieve the same end. The first type of active collector is described by Jacob et al. [97][233]. In this type, fog condenses on the wires, the baffle smoothes the flow of the air, and the fan pulls the air past the wires. Fog droplets collected on the wires move down the wire and are then collected in a clean bottle. The Caltech Active Strand Cloud Collector (CASCC) is another type of active fog collector developed by Daube et al. [98][234] and then modified to include droplet-size fractionation [99][235]. The CASCC has been used in numerous field studies [68][100][101][102][103][104][105][106][107][108][109][16,18,236,237,238,239,240,241,242,243,244]. Fog water droplets are collected by inertial impaction on Teflon strands. The strands are inclined 35 degrees from the vertical. The collected droplets condense together and flow down into a Teflon trough by aerodynamic and gravity forces. Samples are then delivered to the collection bottle. Larger CASCCs are described in Fuzzi et al. [110][245], Minami and Ishizaka [111][246], and Sasakawa and Uematsu [112][247].8. Fog Water Chemistry
Fog consists of water droplets suspended in the air whose diameters typically range from 1 to 100 µm. The LWC is generally smaller than that of rain and cloud waters and varies between 0.01 and 0.5 g m−3. Chemical species found in fogs exist in three phases as gases, interstitial aerosols, and inside the droplet as liquids. The physico-chemical relations among particles, gases, and fog droplets affect fog chemical composition. The incorporation of gases and fine particles into the aqueous phase is a multi-step process. The molecules are first diffused toward the liquid surface where mass transfer across the gas/liquid interface and chemical reactions occur (if any). After that, the species are diffused into the fog droplet [109][113][244,256]. The overall atmospheric concentration of any species “i” in fogs is given by Equation (1) [114][257].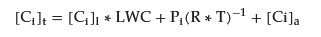 where [Ci]t is the total concentration of any species i (mol m−3), [Ci]l is the concentration of the species “i” in the droplet phase (mol m−3), LWC is the liquid water content (dm3 m−3), Pi is the partial pressure of species “i” in the gas phase (atm), R is the universal gas constant (m3·atm K−1 mol−1), T is the temperature (K), and [Ci]a
is the concentration of the species “i” in the aerosol (mol m−3).
The LWC is an important microphysical parameter that controls fog chemistry. The solute concentration in fog water is proportional to its atmospheric loading but inversely proportional to the LWC which decreases with increasing the LWC. However, instead of falling along a straight line, it has seen an exponential function of the trend. An increase in LWC in fog water leads to a diluting effect of the solute [115][116][117][118][119][120][156,258,259,260,261,262]. However, LWC alone cannot always explain the temporal evolution in terms of the concentrations [109][111][244,246]. The latter is determined by many factors in addition to LWC such as the rate of the chemical reactions, gas scavenging, air masses, and other microphysical properties. Other studies show that no relation exists between LWC and DOC given the differences in gas and particle-phase organic carbon concentrations [109][121][22,244]. During the formation stage, the concentration of pollutants tends to be the highest under the high-temperature inversion where the LWC and the surface area per unit volume (S/V) are the lowest. However, the ratio of surface to LWC is large, meaning higher scavenging efficiency with respect to the dilution effect. Therefore, the concentrations stay at high levels. During the maturation stage, the pollutant concentrations tend to decrease dramatically over the course of the fog event. In this phase, the surface area will be higher leading to an increase in the scavenging potential of pollutants and their subsequent deposition. Further, the LWC also rises with the increase in the supersaturation levels in the maturation period of fog leading to a dilution effect. During the dissipation phase, the surface area will be lower again, meaning that the scavenging potential is lower, and thus, the deposition will be smaller. In addition, the LWC is lower, and the ratio of surface to LWC increases, contributing to a gradual increase in the pollutant concentrations [122][123][227,263].
where [Ci]t is the total concentration of any species i (mol m−3), [Ci]l is the concentration of the species “i” in the droplet phase (mol m−3), LWC is the liquid water content (dm3 m−3), Pi is the partial pressure of species “i” in the gas phase (atm), R is the universal gas constant (m3·atm K−1 mol−1), T is the temperature (K), and [Ci]a
is the concentration of the species “i” in the aerosol (mol m−3).
The LWC is an important microphysical parameter that controls fog chemistry. The solute concentration in fog water is proportional to its atmospheric loading but inversely proportional to the LWC which decreases with increasing the LWC. However, instead of falling along a straight line, it has seen an exponential function of the trend. An increase in LWC in fog water leads to a diluting effect of the solute [115][116][117][118][119][120][156,258,259,260,261,262]. However, LWC alone cannot always explain the temporal evolution in terms of the concentrations [109][111][244,246]. The latter is determined by many factors in addition to LWC such as the rate of the chemical reactions, gas scavenging, air masses, and other microphysical properties. Other studies show that no relation exists between LWC and DOC given the differences in gas and particle-phase organic carbon concentrations [109][121][22,244]. During the formation stage, the concentration of pollutants tends to be the highest under the high-temperature inversion where the LWC and the surface area per unit volume (S/V) are the lowest. However, the ratio of surface to LWC is large, meaning higher scavenging efficiency with respect to the dilution effect. Therefore, the concentrations stay at high levels. During the maturation stage, the pollutant concentrations tend to decrease dramatically over the course of the fog event. In this phase, the surface area will be higher leading to an increase in the scavenging potential of pollutants and their subsequent deposition. Further, the LWC also rises with the increase in the supersaturation levels in the maturation period of fog leading to a dilution effect. During the dissipation phase, the surface area will be lower again, meaning that the scavenging potential is lower, and thus, the deposition will be smaller. In addition, the LWC is lower, and the ratio of surface to LWC increases, contributing to a gradual increase in the pollutant concentrations [122][123][227,263].
