You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Fanny Huang and Version 2 by Fanny Huang.
Adipokinetic hormones (AKHs) regulate important physiological processes in insects. AKHs are short peptides with blocked termini and Trp in position 8. Often, proline occupies position 6. Few post-translational modifications have been found, including hydroxyproline ([Hyp6]) and kynurenine (Kyn). Researchers' latest data suggest that the Hyp- and Kyn-containing AKHs occur more often than originally thought and researchers investigated if they are natural or artifactual. Experimental evidence indicated that Hyp occurs endogenously in insect corpora cardiaca.
- hydroxyproline
- kynurenine
- adipokinetic hormones (AKHs)
1. Introduction
In insects, neuropeptides regulate processes such as homeostasis, development, reproduction, and behavior [1][2]. One of the peptide hormone families, the so-called adipokinetic hormone (AKH)/red pigment-concentrating hormone (RPCH) family, is involved in energy metabolism making stored metabolites available for use in the hemolymph of insects; more than 100 members are known with conserved structural features [3][4]. Mature AKHs are eight to ten amino acids long, with blocked N- (pyroglutamate, pQ) and C- (carboxyamide) termini and specific amino acid residues in each position. Tryptophan is always localized at position 8, and in more than half of all AKHs, a proline residue occupies position 6. A few modifications of AKH peptides have been described, viz. phosphothreonine [5], sulfothreonine [6], C-mannosylation at the tryptophan residue [7][8], proline isomerization (putative) [9], as well as oxidative changes on the Trp and Pro residues, i.e., kynurenine (Kyn) [10] and hydroxyproline (Hyp) [11][12] (Figure 1).
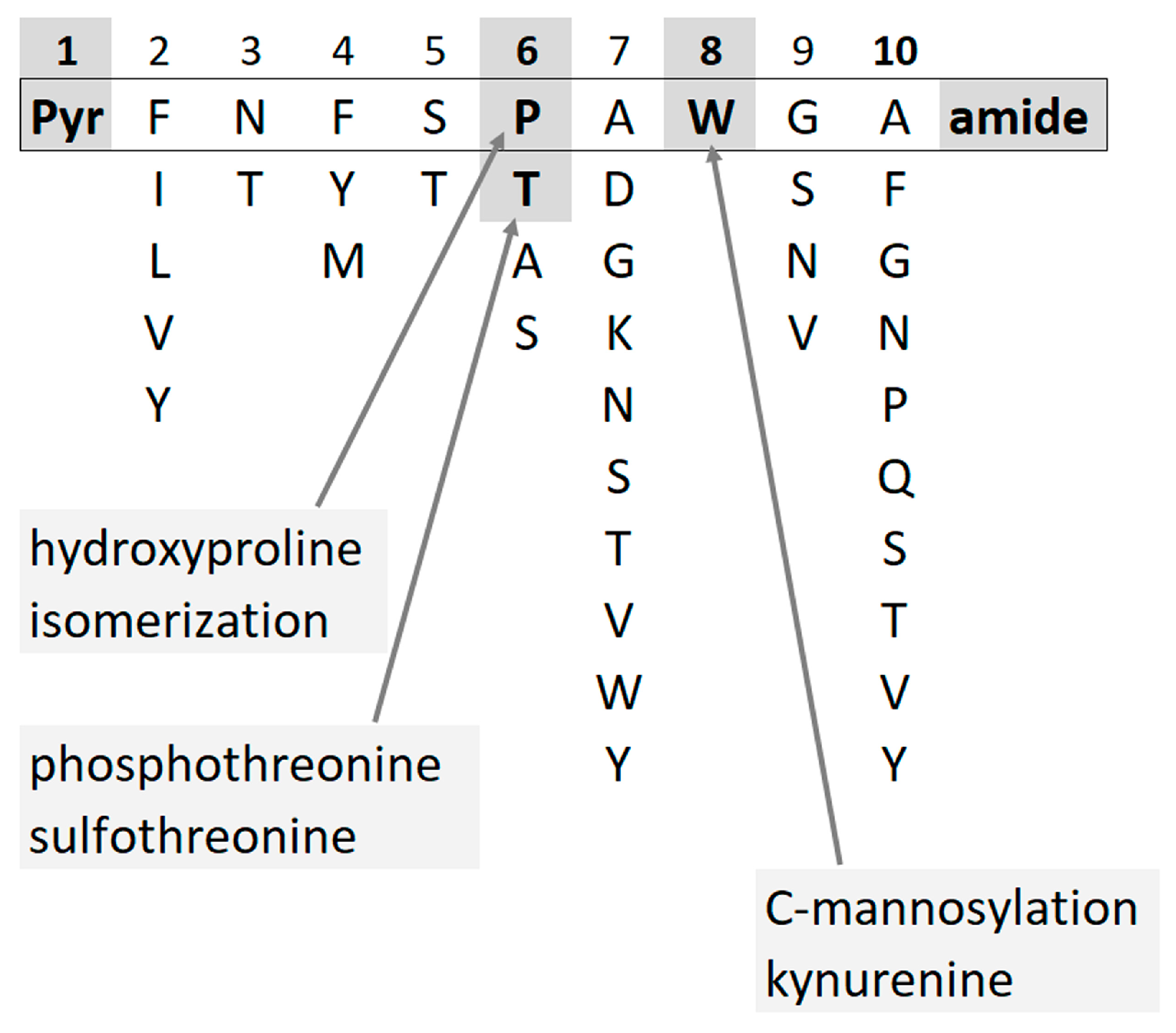
Figure 1. AKHs have conserved structural features such as blocked termini and specific amino acid residues in each position. Few modifications have been described so far.
The finding of a Hyp form of an AKH in diverse insect orders such as Hemiptera and Diptera [11][12] formed the basis of a closer inspection for this peptide modification in subsequent corpora cardiaca (CC) samples. In many cockroach species (order: Blattodea), Hyp-modifications were commonly detected but not verified for most of the [Pro6]-containing AKHs [13].
Trp oxidation of AKHs, on the other hand, is under-reported, but the phenomenon of Trp oxidation in other proteins and peptides may be the result of in vivo processes—some of which may be detrimental to health, some may be neutral, and some may act as a signal (for review, see [14][15]). However, Trp oxidation can also be an artifact from sample handling. It is, therefore, imperative to establish whether an observed modification to a peptide/protein is a natural product or a result of an artificially induced process. Critically, peptide oxidation may occur as an artifact of the ionization process during the electrospray ionization high-resolution mass spectrometry (MS) experiment, and is then detected at the same retention time as the unmodified species [16][17][18]. Naturally, modified peptides (meaning, biologically endogenous as opposed to those with an artificial origin from the MS procedure) will appear at slightly different retention times from those of the unmodified (“parent”) peptide species in liquid chromatography (LC), which separates the analyte peptides before MS.
The biochemical factors behind the oxidative processes that give rise to Hyp and Kyn are supplied below for a better understanding.
2. Hydroxyproline
Hyp is present in animals mainly as trans-4-hydroxy-l-proline and its minor analog trans-3-hydroxy-l-proline in a ratio of ~100:1 [19]. Prolyl hydroxylation is the most common post-translational modification in humans and is known for the stabilizing function of Hyp in the collagen triple helix [20]. The reaction is irreversible and catalyzed by prolyl 4-hydroxylase (P4H), an enzyme that has been described not only in animals but also in plants and microbes (for a review on P4H, see [20]). Hyp is found to a lesser extent in non-collagen proteins; it is of structural and physiological significance, e.g., for scavenging reactive oxygen species (for more information on Hyp structure and function, see [19][21]).
The minimum substrate required for enzyme recognition is described as XPG with the PPG sequence showing the highest hydroxylation rates in some studies [20][22]. In [Ala2]-bradykinin, however, relative hydroxylation was almost three times better for APG than for PPG, and some dependency in oxidation efficiency from N-terminal modifications of the short peptide was observed [23]. In mammalian and frog hypothalamus, [Hyp9]-luteinizing hormone-releasing hormone was detected with the amino acid residue triplet R-Hyp-G [24]. In HeLa S3 cells, besides PPG, also SPG, SPA, TPN, SPE, DPV, and APS sites were hydroxylated [25]. It thus seems that Gly in position 3 of the triplet is not a universal requirement. This was also seen in the case of toxins of the marine gastropod mollusk genus Conus that are reported to contain T-Hyp-Hyp-K, P-Hyp-K, T-Hyp-P, T-Hyp-Hyp-R, P-Hyp-R, K-Hyp-Q, R-Hyp-T, and D-Hyp-R [26][27][28].
Little is known about hydroxyprolination in insects. The first report of a hydroxyprolinated AKH ([Hyp6]-Panbo-RPCH, code-named Nezvi-AKH) was in 2011 from the CC of the green stink bug Nezara viridula, with Panbo-RPCH (pQLNFSPGW amide) as the “parent peptide” being modified [11]. Eleven years later, this modification was shown for an AKH in the horse fly Haematopota pluvialis: the hydroxyprolinated Tabat-AKH (pQLTFTP GW amide) was code-named Haepl-AKH [12]). Extensive experiments with Nezvi-AKH, including extraction in an oxygen-free atmosphere, were performed to exclude artifactual Pro oxidation [11]; such checks were not carried out with Haepl-AKH.
There is, however, not much evidence in the literature that Hyp is easily formed at ambient conditions—in contrast to spontaneous methionine oxidation during sample handling, which has been abundantly proven for peptides and proteins [29]. Apparently, the presence at least of oxidizing agents is required to modify Pro; this residue in human apolipoprotein B-100 was highly reactive toward oxygen radicals in vitro for two different oxidation systems in the presence of low iron concentrations [30]. Radical attack (copper and H2O2) also modified Pro residues in a cell lysate of primary cultures of chick embryo myotubes [31], but in these experiments, the involvement of enzymes could not be excluded.
Using the search term “prolyl hydroxylase”, wresearchers extracted 750 entries for this enzyme in insects from the Uniprot protein database. Except for one result (Q9I7H9, Drosophila melanogaster), they were all unreviewed entries, which were assigned to the enzyme class by sequence similarity to known proteins with little experimental backing. D. melanogaster sudestada1 (sud1), however, was identified as a gene that is needed for normal growth in the fly [32]. Sud1 encodes a prolyl-hydroxylase that catalyzes post-translational hydroxylation of a conserved residue in the small ribosomal subunit protein RPS23; knockdown of Sud1 results in growth impairment and reduced RPS23 hydroxylation, which is associated with activation of the unfolded protein response, induction of apoptosis, and increased autophagy [32].
3. Tryptophan Oxidation
Ten years ago, a Kyn-containing variant of an AKH formerly identified as a hypertrehalosemic hormone in the Indian stick insect Carausius morosus (Carmo-HrTH [33]) was detected as pQLTFTPN-Kyn-GT amide in the Vietnamese stick insect, Baculum extradentatum [10]. At the time, it was thought to be a post-translational modification, but mounting evidence and the present study suggest that it is more likely to be a handling artifact. The indole ring in Trp is highly reactive (primarily the pyrrole ring [34]) and this amino acid residue is susceptible to oxidation and degradation into multiple products during sample preparation [35]. Factors like reactive oxygen species (singlet oxygen, hydrogen peroxide, hydroxyl radicals), light and photosensitizers, metals, and heat may contribute to these processes (for an introductory review, see [35]). The stability of Trp-containing products is a well-known problem in the food (e.g., milk proteins [36][37]) and textile industries [38] as well as for pharmacological preparations (e.g., monoclonal antibodies [39]). Kyn is a common oxidation product in addition to singly, doubly, and triply oxidated forms (Figure 2), but even more Trp modifications have been described [35][40].
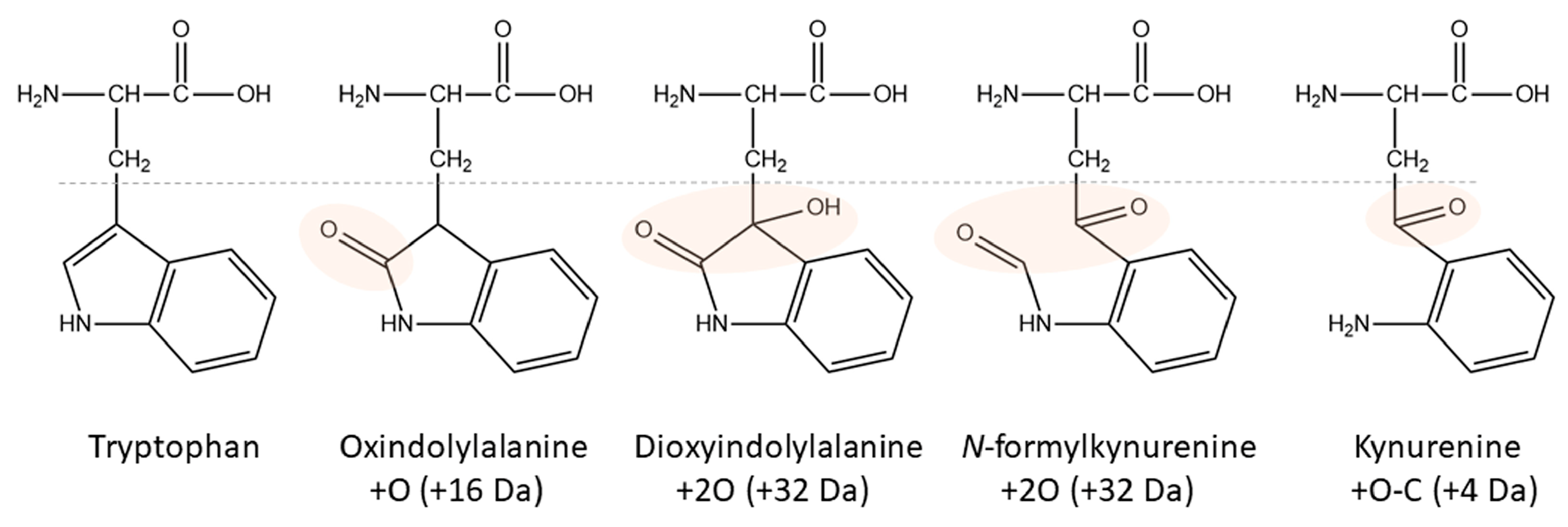
Figure 2. Structures of Trp and its major oxidation products. Modified areas are highlighted. The corresponding nominal mass increase is given in brackets.
In an earlier investigation, difficulties arose when studying the actions of α-melanocyte stimulating hormone (αMSH) and αMSH(1-12) (AcSYSMEHFRWGKPV amide) in cell culture, because the Trp residues of the hormone were oxidized to at least five different products [41]. Peptide oxidation was slowed by the addition of tris-(2-chloroethyl) phosphate, a very effective reducing agent, but its use was limited by its cell toxicity at higher concentrations. Interestingly, no special chemicals such as hydrogen peroxide or irradiation (as found in other studies: photooxidation [40][42][43], ozone [44][45], and gel electrophoresis procedures [46]) were necessary to modify the Trp residue in experimentation with αMSH and αMSH(1-12); it sufficed to keep the peptides at room temperature for some time [41]. Thereby, Trp oxidation took longer than Met oxidation and was not as dominant at higher peptide concentrations.
Trp oxidation has also been described in in vivo processes. For instance, exposure of Arabidopsis thaliana plants to light stress resulted in an increased level of oxidized Trp in proteins of the photosystem II reaction center and the oxygen-evolving complex [47]. Increased levels of oxidative stress are also believed to play a key role in the development of age-related diseases through the oxidation of amino acid residues, and this was substantiated for Trp in α-skeletal actin and troponin I using a rat model of acute oxidative stress induced by X-ray irradiation [48]. Both of these studies, however, used gel electrophoresis for protein separation before analysis, which was shown to oxidize Trp [46]. In other work, modified Trp residues were detected with LC-MS in cyclic microcystin peptides prepared from cyanobacterium Microcystis sp. CAWBG11 using precautions to avoid artificial oxidation [49].
Trp oxidation does not always require an enzyme to catalyze it, although such processes are also known from the Kyn pathway. Tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase are members of a small family of heme enzymes that catalyze the aerobic metabolism of L-Trp to N-formylkynurenine in both eukaryotes and prokaryotes [50]. While these two enzymes have only been described to act on Trp or some of its small molecule derivatives, a peptide-tryptophan 2,3-dioxygenase, also called pyrrolooxygenase (PO), has been reported from plant and animal sources, which forms peptide formylkynurenine [51][52]. For TDO in insects, several entries were found in Uniprot, such as P20351 for D. melanogaster and Q17P71 for Aedes aegypti, but none for PO.
Thus, the aims of the related study (König, S. et al. [53]) were: (1) to validate the [Hyp6]-modified AKH sequences recently observed in cockroaches [13] with the use of synthetic peptides and LC-MS, (2) to ascertain if these peptides retain biological activity in a metabolic assay, and (3) to elucidate whether the prevalence of the modification is an artifact arising from peptide handling, or whether the oxidated form is already present in the CC itself (and, thus, releasable ex vivo). Furthermore, (4) the investigations to detect other oxidized AKHs, specifically Trp oxidation, were extended to beetles (Order: Coleoptera) through the availability of CC extracts from burying beetle species of the genus Nicrophorus.
References
- Nässel, D.R.; Winther, Å. Drosophila neuropeptides in regulation of physiology and behavior. Prog. Neurobiol. 2010, 92, 42–104.
- Gäde, G.; Hoffmann, K.H.; Spring, J.H. Hormonal regulation in insects: Facts, gaps, and future directions. Physiol. Rev. 1997, 77, 963–1032.
- Gäde, G.; Marco, H.G. Structure, function and mode of action of select arthropod neuropeptides. In Bioactive Natural Products (Part M); Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 69–139.
- Marco, H.G.; Gäde, G. Adipokinetic hormone: A hormone for all seasons? In Advances in Invertebrate (Neuro)Endocrinology: A Collection of Reviews in the Post-Genomic Era; Saleuddin, S., Lange, A.B., Orchard, I., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2020; Volume 2, pp. 126–170.
- Gäde, G.; Šimek, P.; Clark, K.D.; Auerswald, L. Unique translational modification of an invertebrate neuropeptide: A phosphorylated member of the adipokinetic hormone peptide family. Biochem. J. 2006, 393, 705–713.
- Gäde, G.; Šimek, P.; Marco, H.G. A sulfothreonine adipokinetic peptide—A novel post-translational modification revealed in the twig wilter Holopterna alata (Hemiptera, Coreidae). In Proceedings of the 27th Conference of European Comparative Endocrinologists, Rennes, France, 25–29 August 2014; p. 68.
- Munte, C.E.; Gäde, G.; Domogalla, B.; Kremer, W.; Kellner, R.; Kalbitzer, H.R. C-mannosylation in the hypertrehalosaemic hormone from the stick insect Carausius morosus. FEBS J. 2008, 275, 1163–1173.
- Gäde, G.; Marco, H.G. The unique C-mannosylated hypertrehalosemic hormone of Carausius morosus: Identity, release, and biological activity. Arch. Insect Biochem. Physiol. 2023, 113, e22016.
- König, S.; Bayer, M.; Marco, H.G.; Gäde, G. The hypertrehalosaemic neuropeptide conformational twins of cicadas consist of only L-amino acids: Are they cis–trans isomers? Amino Acids 2019, 51, 1023–1028.
- Malik, A.; Gäde, G.; Lange, A.B. Sequencing and biological effects of an adipokinetic/hypertrehalosemic peptide in the stick insect, Baculum extradentatum. Peptides 2012, 34, 51–56.
- Gäde, G.; Šimek, P.; Marco, H.G. An invertebrate -modified neuropeptide: Further evidence for a close evolutionary relationship between insect adipokinetic hormone and mammalian gonadotropin hormone family. Biochem. Biophys. Res. Commun. 2011, 414, 592–597.
- Marco, H.G.; König, S.; Gäde, G. Mass spectrometric proof of predicted peptides: Novel adipokinetic hormones in insects. Molecules 2022, 27, 6469.
- Marco, H.G.; König, S.; Gäde, G. Predicted novel hypertrehalosaemic peptides of cockroaches are verified by mass spectrometry. Amino Acids 2023, 55, 1641–1654.
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228.
- Davies, M.J.; Truscott, R.J.W. Chapter 12—Photo-oxidation of proteins and its consequences. In Comprehensive Series in Photosciences; Giacomoni, P.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 251–275.
- Chen, M.; Cook, K.D. Oxidation artifacts in the electrospray mass spectrometry of Aβ peptide. Anal Chem. 2007, 79, 2031–2036.
- Morand, K.; Talbo, G.; Mann, M. Oxidation of peptides during electrospray ionization. Rapid Commun. Mass Spectrom. 1993, 7, 738–743.
- Schweikart, F.; Hulthe, G. HPLC-UV-MS analysis: A source for severe oxidation artifacts. Anal. Chem. 2019, 91, 1748–1751.
- Hu, S.; He, W.; Wu, G. Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids 2022, 54, 513–528.
- Gorres, K.L.; Raines, R.T. Prolyl 4-hydroxylase. Crit. Rev. Mol. Biol. 2010, 45, 106–124.
- Zahradníčková, H.; Opekar, S.; Řimnáčová, L.; Šimek, P.; Moos, M. Chiral secondary amino acids, their importance, and methods of analysis. Amino Acids 2022, 54, 687–719.
- Rapaka, R.S.; Renugopalakrishnan, V.; Urry, D.W.; Bhatnagar, R.S. Hydroxylation of proline in polytripeptide models of collagen: Stereochemistry of polytripeptide-prolyl hydroxylase interaction. Biochemistry 1978, 17, 2892–2898.
- McGee, J.O.; Rhoads, R.E.; Udenfriend, S. The substrate recognition site of collagen proline hydroxylase: The hydroxylation of -X-Pro-Gly- sequences in bradykinin analogs and other peptides. Arch. Biochem. Biophys. 1971, 144, 343–351.
- Gautron, J.P.; Pattou, E.; Bauer, K.; Kordon, C. (Hydroxyproline(9)) luteinizing hormone-releasing hormone: A novel peptide in mammalian and frog hypothalamus. Neurochem. Int. 1991, 18, 221–235.
- Arsenault, P.R.; Heaton-Johnson, K.J.; Li, L.S.; Song, D.; Ferreira, V.S.; Patel, N.; Master, S.R.; Lee, F.S. Identification of prolyl hydroxylation modifications in mammalian cell proteins. Proteomics 2015, 15, 1259–1267.
- Cruz, L.J.; Gray, W.R.; Olivera, B.M.; Zeikus, R.D.; Kerr, L.; Yoshikami, D.; Moczydlowski, E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J. Biol. Chem. 1985, 260, 9280–9288.
- Buczek, O.; Bulaj, G.; Olivera, B.M. Conotoxins and the posttranslational modification of secreted gene products. Cell. Mol. Life Sci. 2005, 62, 3067–3079.
- Zhang, B.-B.; Zhao, C.; Wang, X.-S.; He, L.; Du, W.-H. Effects of 4-hydroxyproline stereochemistry on α-conotoxin solution conformation. Acta Phys. Chim. Sin. 2013, 29, 1080–1087.
- Kim, G.; Weiss, S.J.; Levine, R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta 2014, 1840, 901–905.
- Pietzsch, J. Measurement of 5-hydroxy-2-2aminovaleric acid as a specific marker of iron-mediated oxidation of proline and arginine side-chain residues of low-density lipoprotein apolipoprotein B-100. Biochem. Biophys. Res. Commun. 2000, 270, 852–857.
- Dean, R.T.; Wolff, S.P.; McElligott, M.A. Histidine and proline are important sites of free radical damage to proteins. Free Rad. Res. Comms. 1989, 7, 97–103.
- Katz, M.J.; Acevedo, J.M.; Loenarz, C.; Galagovsky, D.; Liu-Yi, P.; Pérez-Pepe, M.; Thalhammer, A.; Sekirnik, R.; Ge, W.; Melani, M.; et al. Sudestada1, a Drosophila ribosomal prolyl-hydroxylase required for mRNA translation, cell homeostasis, and organ growth. Proc. Natl. Acad. Sci. USA 2014, 111, 4025–4030.
- Gäde, G. Peptides of the adipokinetic hormone/red pigment-concentrating hormone family—A new take on biodiversity. In Trends in Comparative Endocrinology and Neurobiology; Annals of the New York Academy of Science; Vaudry, H., Roubos, E.W., Coast, G.M., Vallarino, M., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2009; Volume 1163, pp. 125–136.
- Simat, T.J.; Steinhart, H. Oxidation of free tryptophan and tryptophane residues in peptides and proteins. J. Agric. Food Chem. 1998, 46, 490–498.
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 20, 696–718.
- Scheidegger, D.; Larsen, G.; Kivatinitz, S.C. Oxidative consequences of UV irradiation on isolated milk proteins: Effects of hydrogen peroxide and bivalent metal ions. Int. Dairy J. 2016, 55, 64–71.
- Koivumäki, T.P.; Gürbüz, G.; Heinonen, I.M. Tryptophan and cysteine oxidation products dominate in α-lactalbumin-derived peptides analyzed with LC-MSn. J. Food Sci. 2017, 82, 2062–2069.
- Dyer, J.M.; Bringans, S.D.; Bryson, W.G. Characterisation of photo-oxidation products within photoyellowed wool proteins: Tryptophan and tyrosine derived chromophores. Photochem. Photobiol. Sci. 2006, 5, 698–706.
- Barnett, G.V.; Balakrishnan, G.; Chennamsetty, N.; Hoffman, L.; Bongers, J.; Tao, L.; Huang, Y.; Slaney, T.; Das, T.K.; Leone, A.; et al. Probing the tryptophan environment in therapeutic proteins: Implications for higher order structure on tryptophan oxidation. J. Pharm. Sci. 2019, 108, 1944–1952.
- Grosvenor, A.J.; Morton, J.D.; Dyer, J.M. Profiling of residue-level photo-oxidative damage in peptides. Amino Acids 2010, 39, 285–296.
- Bayer, M.; Tsiskarishvili, N.; Stegemann, A.; Böhm, M.; König, S. Fast oxidation of α-melanocyte stimulating hormone and derived peptides under laboratory conditions causes irreproducible results—Insights from studies of prolylcarboxypeptidase in human cell types. Pigment. Cell Melanoma Res. 2019, 33, 378–382.
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53.
- Castaño, C.; Vignoni, M.; Vicendo, P.; Oliveros, E.; Thomas, A.H. Degradation of tyrosine and tryptophan residues of peptides by type I photosensitized oxidation. J. Photochem. Photobiol. B Biol. 2016, 164, 226–235.
- Cohen, S.L. Ozone in ambient air as a source of adventitious oxidation. A mass spectrometric study. Anal. Chem. 2006, 78, 4352–4362.
- Kotiaho, T.; Eberlin, M.N.; Vainiotalo, P.; Kostiainen, R. Electrospray mass and tandem mass spectrometry identification of ozone oxidation products of amino acids and small peptides. J. Am. Soc. Mass Spectrom. 2000, 11, 526–535.
- Perdivara, I.; Deterding, L.J.; Przybylski, M.; Tomer, K.B. Mass spectrometric identification of oxidative modifications of tryptophan residues in proteins: Chemical artifact or posttranslational modification? J. Am. Soc. Mass Spectrom. 2010, 21, 1114–1117.
- Galetskiy, D.; Lohscheider, J.N.; Kononikhin, A.S.; Popov, I.A.; Nikolaev, E.N.; Adamska, I. Mass spectrometric characterization of photooxidative protein modifications in Arabidopsis thaliana thylakoid membranes. Rapid Commun. Mass Spectrom. 2011, 25, 184–190.
- Fedorova, M.; Todorovsky, T.; Kuleva, N.; Hoffmann, R. Quantitative evaluation of tryptophan oxidation in actin and troponin I from skeletal muscles using a rat model of acute oxidative stress. Proteomics 2010, 10, 2692–2700.
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Miles, C.O.; Rise, F.; Cary, S.C.; Hamilton, D.P.; Wilkins, A.L. Structural characterization of new microcystins containing tryptophan and oxidized tryptophan residues. Mar. Drugs. 2013, 11, 3025–3045.
- Thackray, S.J.; Mowat, C.G.; Chapman, S.K. Exploring the mechanism of tryptophan 2,3-dioxygenase. Biochem. Soc. Trans. 2008, 36, 1120–1123.
- Frydman, R.B.; Tomaro, M.L.; Frydman, B. Pyrrolooxygenase: Its action on tryptophan-containing enzymes and peptides. Biochim. Biophys. Acta 1972, 284, 80–89.
- Frydman, B.; Frydman, R.B.; Tomaro, M.L. Pyrrolooxygenases: A new type of oxidases. Mol. Cell Biochem. 1973, 2, 121–136.
- König, S.; Marco, H.G.; Gäde, G. Oxidation Products of Tryptophan and Proline in Adipokinetic Hormones—Artifacts or Post-Translational Modifications? Life 2023, 13, 2315.
More
