Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Wojciech Kazimierczak.
Abdominal aortic aneurysms (AAAs) are a significant cause of mortality in developed countries. Endovascular aneurysm repair (EVAR) is currently the leading treatment method for AAAs. Due to the high sensitivity and specificity of post-EVAR complication detection, CT angiography (CTA) is the reference method for imaging surveillance in patients after EVAR. Many studies have shown the advantages of dual-energy CT (DECT) over standard polyenergetic CTA in vascular applications.
- dual-energy computed tomography
- endoleaks
- abdominal aortic aneurysm
- virtual monoenergetic images
- metal artifact reduction
Applications of DECT in Patients after EVAR
1.1. Radiation Dose Reduction
1. Radiation Dose Reduction
The ionizing radiation dose associated with lifelong diagnostic surveillance is a fundamental problem related to post-endovascular aneurysm repair (EVAR) follow-up protocols. The risk of radiation-induced cancer related to repeated CT scans is already well established [34,35,36,37,38][1][2][3][4][5]. The basic methods of radiation dose reduction are automatic exposure systems, iterative techniques, and regular service of the tomographic device [39][6]. One way to reduce the radiation dose is to lower the tube current and voltage; however, this increases the image noise and decreases the diagnostic value of the examination [40][7].
Among the concerns regarding dual-energy CT examinations, the most significant and recurring are those related to radiation dose. Several studies have demonstrated that the dose delivered during dual-source dual-energy CT acquisition is similar to that of comparable single-energy CT (SECT) [41,42,43,44][8][9][10][11]. With the advancement of technology, the introduction of iterative techniques, improved detector efficiency, and spectral filtration systems have made it possible to deliver even lower radiation doses with DECT than with SECT [26,45][12][13].
Several researchers have highlighted the possibility of using shortened examinations, limited to phases performed after administering a contrast agent, with VNC phase reconstruction without a significant reduction in the sensitivity of CTA for detecting endoleaks [46,47,48,49,50,51][14][15][16][17][18][19]. The dose reduction obtained in these studies primarily results from skipping the native phase of the examination. The aforementioned studies also pointed out the possibility of dose reduction by additionally skipping the arterial phase of the examination while maintaining the high sensitivity of the single-phase protocol for detecting endoleaks. A summary of these studies is provided in Table 1.
Table 1.
Reduction in the average radiation dose in DECT studies compared with the triphasic examination protocol.
| Research | Protocol | Dose Reduction (%) | |
|---|---|---|---|
| Mono-Phasic (mSv) | Three-Phasic (mSv) | ||
| Chandarana et al., 2008 [47][15] | 11.1 | 27.8 | 61 |
| Flors et al., 2013 [48][16] | 9.8 | 22.4 | 64.1 |
| Stolzman et al., 2008 [49][17] | 10.9 | 27.4 | 61 |
| Buffa et al., 2014 [50][18] | 10.5 | 27.4 | 61.7 |
| Kazimierczak et al., 2023 [51][19] | 10.69 | 27.96 | 61.37 |
Since cumulative radiation exposure is a fundamental factor influencing post-EVAR diagnostic surveillance protocols, CTA protocols are a compromise between radiation dose and diagnostic accuracy. There is generally a consensus among researchers regarding reducing the number of examination phases by omitting the native phase and reconstructing the VNC. However, the number of examination phases performed after administering a contrast agent is controversial. A few authors have highlighted the importance of the arterial phase in detecting endoleaks, demonstrating a higher sensitivity of multiphasic (VNC + 2 postcontrast DECT acquisitions) examination protocols [48,51][16][19]. Potentially life-threatening and requiring treatment, type I and type III endoleaks require the arterial phase of examination for diagnosis [52][20]. Despite discrepancies in the literature on the necessity of the arterial phase for detecting endoleaks, this phase unquestionably holds value in assessing the potential narrowing of abdominal arteries [53,54][21][22]. This issue is particularly significant in the case of post-br/fEVAR procedures due to a higher risk of complications within the target arteries. Furthermore, the arterial phase allows for the evaluation of perfusion disorders of the abdominal organs and potentially the implementation of appropriate treatment. In the general elderly population of EVAR patients, acquiring the arterial phase might be particularly important in assessing additional findings such as tumors. Therefore, the presence of the arterial phase in CTA protocols appears justified. However, the optimal scanning protocol for CT scanning remains controversial [48,49,51,55,56,57][16][17][19][23][24][25].
An interesting study in this context was conducted by Javor et al., who demonstrated the possibility of reducing the radiation dose by 42% via a split-bolus technique with one DE acquisition and VNC reconstruction with 96% sensitivity in endoleak detection [58][26]. Similar results were achieved by Boos and Iezzi [59,60][27][28]. In theory, this technique allows for the optimal contrast of low- and high-flow endoleaks, as well as arterial vessels and parenchymal organs, during a single scan. However, despite promising results, the literature lacks sufficient evidence for the effectiveness of split-bolus protocols in post-EVAR surveillance.
1.2. Contrast Agent Volume Reduction
2. Contrast Agent Volume Reduction
The use of contrast agents is mandatory in both procedure planning and post-EVAR diagnostic surveillance. The accuracy of delineating three-dimensional vessel structures is crucial for the selection of proper surgical devices and the diagnosis of postprocedural complications. However, high-quality CTA requires the administration of an appropriate volume of iodine contrast agent. The use of contrast agents is associated with adverse effects, such as hypersensitivity allergic reactions, thyroid dysfunction, and nephropathy [61,62,63][29][30][31]. To minimize the risk of contrast media-induced nephropathy, it is recommended to use as o.w. volume of a contrast agent as possible for diagnostic imaging [64,65,66][32][33][34]. Therefore, contrast agent volume reduction techniques are used.
The phenomenon of high CT attenuation numbers in low-level virtual monoenergetic images (VMIs) is well established [25,67][35][36]. When low-level VMIs are used, CT attenuation of the contrast material can be increased, allowing for the injection of a lower dose of iodine [68][37]. Low-level VMIs have been shown to boost vascular contrast in several vascular beds, which can reduce the volume of the contrast agent [69,70,71][38][39][40]. Studies have shown that necessary preprocedural measurements of the aorta can be acquired with low-level VMIs, permitting imaging with an equivalent radiation dose but a lower contrast dose than standard SECT [72,73][41][42].
Currently, there is a lack of data in the literature on the diagnostic accuracy of protocols involving reduced contrast agent administration. Despite this, this issue is of significant importance in the context of follow-up CTA of EVAR patients. In clinical practice, there are instances of administering reduced amounts of contrast agents due to staff errors, disconnection of injecting system components, access vessel rupture, or incorrect acquisition timing. Additionally, radiological protection concerns, such as avoiding the repetition of poorly performed examinations, justify the need to implement methods that allow for the assessment of CTA with suboptimal vessel enhancement. Low-energy VMIs may enable a reduction in rejected examinations and provide a reliable assessment of endoleaks in these specific clinical settings. Figure 1 shows the differences between conventional, linearly blended, and low-level VMI reconstructions in type 3 endoleaks.
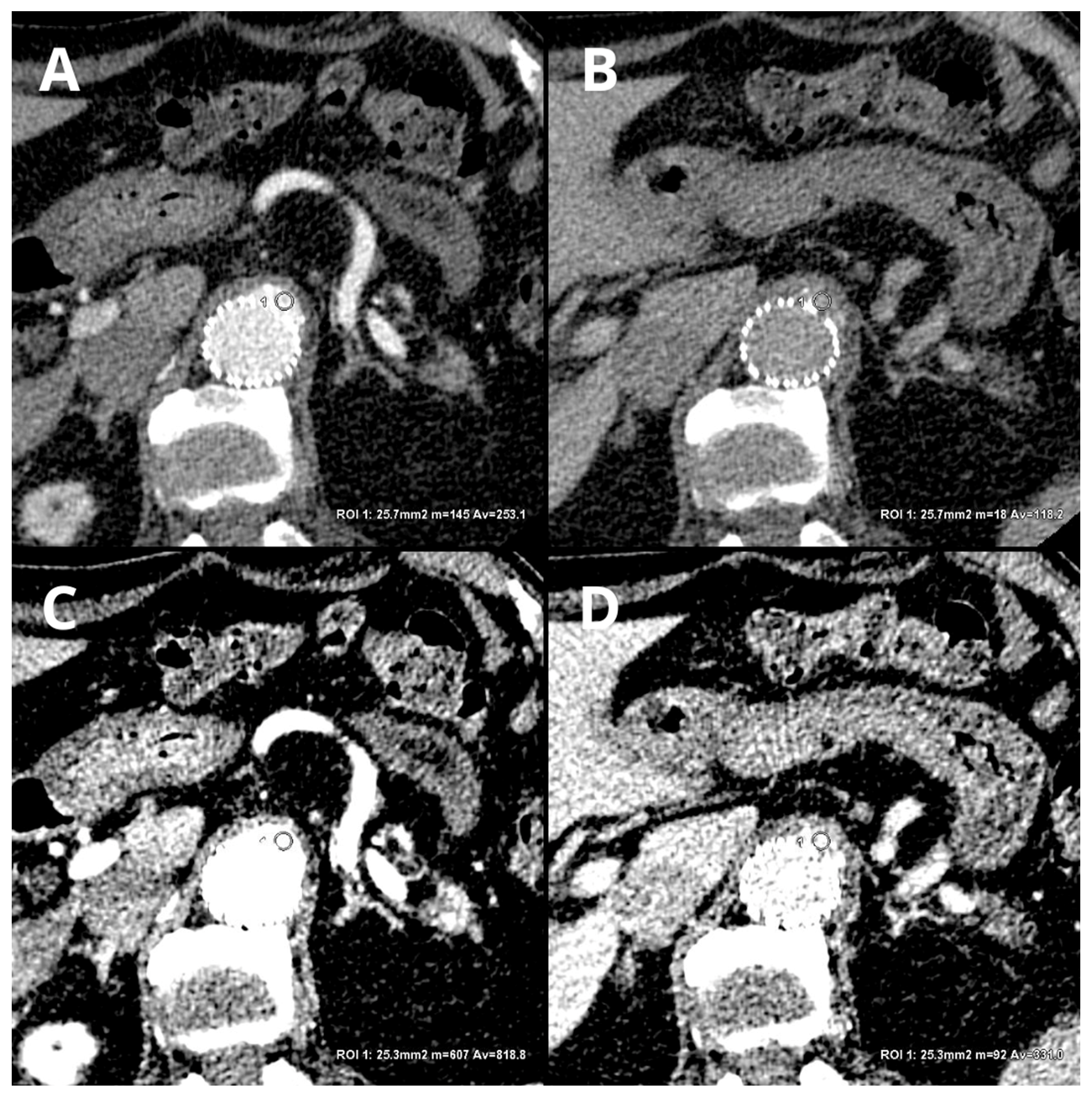
Figure 1. Comparison of LB and 40 keV VMI reconstructions in arterial and delayed phases: LB arterial ((A)—253.1 ± 50.2 HU), LB delayed ((B)—118.2 ± 25.4), 40 keV VMI arterial ((C)—818.8 ± 116.5 HU), and 40 keV VMI delayed ((D)—331 ± 53.4 HU). An automatic region-of-interest (ROI) propagation tool was used. The same window settings (W 500, L 100) were used to highlight the differences in the contrast visualization. LB-delayed and 40 keV VMI-delayed images serve as examples of the potential to salvage an examination with a reduced volume of contrast agent.
1.3. Endoleak Detection
3. Endoleak Detection
Low-level VMIs are a major factor in the superiority of DECT over SECT for detecting endoleaks. To date, few studies have assessed the impact of low-level VMIs on the diagnostic accuracy of endoleak detection [51,74,75,76][19][43][44][45]. A study by Maturen et al. [74][43] showed a higher sensitivity for endoleak detection with a VMI of 55 keV than a VMI of 75 keV. Martin et al. [75][44] reported a significantly greater rate of endoleak detection in VMI and VMI+ compared to standard linearly blended images (LB). These results were accompanied by a significant improvement in the image quality parameters (contrast-to-noise ratio). Comparable results were achieved by Kazimierczak et al. [51][19] in a 2023 study, showing a significant increase in the number of endoleaks diagnosed (an increase of almost 30%) and an improvement in image quality parameters with 40 keV VMI compared to LB images. Charalombous et al. reported the use of a 54 keV VMI to enhance the efficiency of endoleak detection efficiency. Moreover, analysis of the normalized effective atomic number and improvised endoleak index was found to have significant power in predicting the aggressiveness of type II endoleaks [77][46]. However, all of the mentioned studies were conducted on relatively small study groups with fewer than 100 patients and did not influence the current guidelines regarding post-EVAR follow-up. An interesting study by Skawran et al. [76][45] compared low-level VMIs and single-energy low-kV images (SEIs) in terms of the diagnostic accuracy of six readers in endoleak detection as well as subjective and objective image quality properties. The results of this restudy earch indicated that a low-keV VMI+ improved the contrast-to-noise ratio of the aorta. However, the noise level, subjective image quality, and diagnostic accuracy of endoleaks were superior for SEI. Although the results of the present study are related to analyses performed on a phantom, they suggest a promising direction for further research to improve the detectability of endoleaks.
References
- Dixon, A.K.; Dendy, P. Spiral CT: How much does radiation dose matter? Lancet 1998, 352, 1082–1083.
- Einstein, A.J.; Henzlova, M.J.; Rajagopalan, S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007, 298, 317–323.
- de Jong, P.A.; Mayo, J.R.; Golmohammadi, K.; Nakano, Y.; Lequin, M.H.; Tiddens, H.A.W.M.; Aldrich, J.; Coxson, H.O.; Sin, D.D. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am. J. Respir. Crit Care Med. 2006, 173, 199–203.
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284.
- Brenner, D.J.; Elliston, C.D. Estimated radiation on risks potentially associated with full-body CT screening. Radiology 2004, 232, 735–738.
- White, H.A.; MacDonald, S. Estimating risk associated with radiation exposure during follow-up after endovascular aortic repair (EVAR). J. Cardiovasc. Surg. 2010, 51, 95.
- Lehti, L.; Nyman, U.; Söderberg, M.; Björses, K.; Gottsäter, A.; Wassélius, J. 80-kVp CT angiography for endovascular aneurysm repair follow-up with halved contrast medium dose and preserved diagnostic quality. Acta Radiol. 2016, 57, 279–286.
- Grajo, J.R.; Sahani, D.V. Dual-Energy CT of the Abdomen and Pelvis: Radiation Dose Considerations. J. Am. Coll. Radiol. 2018, 15, 1128–1132.
- Weinman, J.P.; Mirsky, D.M.; Jensen, A.M.; Stence, N.V. Dual energy head CT to maintain image quality while reducing dose in pediatric patients. Clin. Imaging 2019, 55, 83–88.
- Siegel, M.J.; Curtis, W.A.; Ramirez-Giraldo, J.C. Effects of dual-energy technique on radiation exposure and image quality in pediatric body CT. Am. J. Roentgenol. 2016, 207, 826–835.
- Goo, H.W. Initial experience of dual-energy lung perfusion CT using a dual-source CT system in children. Pediatr. Radiol. 2010, 40, 1536–1544.
- Siegel, M.J.; Ramirez-Giraldo, J.C. Dual-energy CT in children: Imaging algorithms and clinical applications. Radiology 2019, 291, 286–297.
- Primak, A.N.; Giraldo, J.C.R.; Eusemann, C.D.; Schmidt, B.; Kantor, B.; Fletcher, J.G.; McCollough, C.H. Dual-source dual-energy CT with additional tin filtration: Dose and image quality evaluation in phantoms and in vivo. Am. J. Roentgenol. 2010, 195, 1164–1174.
- Macari, M.; Chandarana, H.; Schmidt, B.; Lee, J.; Lamparello, P.; Babb, J. Abdominal aortic aneurysm: Can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology 2006, 241, 908–914.
- Chandarana, H.; Godoy, M.C.B.; Vlahos, I.; Graser, A.; Babb, J.; Leidecker, C.; Macari, M. Abdominal aorta: Evaluation with dual-source dual-energy multidetector CT after endovascular repair of aneurysms-initial observations. Radiology 2008, 249, 692–700.
- Flors, L.; Leiva-Salinas, C.; Norton, P.T.; Patrie, J.T.; Hagspiel, K.D. Endoleak detection after endovascular repair of thoracic aortic aneurysm using dual-source dual-energy CT: Suitable scanning protocols and potential radiation dose reduction. Am. J. Roentgenol. 2013, 200, 451–460.
- Stolzmann, P.; Frauenfelder, T.; Pfammatter, T.; Peter, N.; Scheffel, H.; Lachat, M.; Schmidt, B.; Marincek, B.; Alkadhi, H.; Schertler, T. Endoleaks after endovascular abdominal aortic aneurysm repair: Detection with dual-energy dual-source CT. Radiology 2008, 249, 682–691.
- Buffa, V.; Solazzo, A.; D’auria, V.; Del Prete, A.; Vallone, A.; Luzietti, M.; Madau, M.; Grassi, R.; Miele, V. Dual-source dual-energy CT: Dose reduction after endovascular abdominal aortic aneurysm repair. Radiol. Medica 2014, 119, 934–941.
- Kazimierczak, W.; Kazimierczak, N.; Lemanowicz, A.; Nowak, E.; Migdalski, A.; Jawien, A.; Jankowski, T.; Serafin, Z. Improved Detection of Endoleaks in Virtual Monoenergetic Images in Dual-Energy CT Angiography Following EVAR. Acad. Radiol. 2023, 30, 2813–2824.
- Iezzi, R.; Cotroneo, A.R.; Filippone, A.; Di Fabio, F.; Quinto, F.; Colosimo, C.; Bonomo, L. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: Are unenhanced and delayed phase enhanced images effective for endoleak detection? Radiology 2006, 241, 915–921.
- Glebova, N.O.; Selvarajah, S.; Orion, K.C.; Black, J.H.; Malas, M.B.; Perler , B.A.; Abularrage , C.J. Fenestrated endovascular repair of abdominal aortic aneurysms is associated with increased morbidity but comparable mortality with infrarenal endovascular aneurysm repair. J. Vasc. Surg. 2015, 61, 604–610.
- Troisi, N.; Donas, K.P.; Austermann, M.; Tessarek, J.; Umscheid, T.; Torsello, G. Secondary procedures after aortic aneurysm repair with fenestrated and branched endografts. J. Endovasc. Ther. 2011, 18, 146–153.
- Sommer, W.H.; Becker, C.R.; Haack, M.; Rubin, G.D.; Weidenhagen, R.; Schwarz, F.; Nikolaou, K.; Reiser, M.F.; Johnson, T.R.; Clevert, D.A. Time-resolved CT angiography for the detection and classification of endoleaks. Radiology 2012, 263, 917–926.
- Stavropoulos, S.W.; Charagundla, S.R. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 2007, 243, 641–655.
- Iezzi, R.; Cotroneo, A.R.; Filippone, A.; Santoro, M.; Basilico, R.; Storto, M.L. Multidetector-row computed tomography angiography in abdominal aortic aneurysm treated with endovascular repair: Evaluation of optimal timing of delayed phase imaging for the detection of low-flow endoleaks. J. Comput. Assist. Tomogr. 2008, 32, 609–615.
- Javor, D.; Wressnegger, A.; Unterhumer, S.; Kollndorfer, K.; Nolz, R.; Beitzke, D.; Loewe, C. Endoleak detection using single-acquisition split-bolus dual-energy computer tomography (DECT). Eur. Radiol. 2017, 27, 1622–1630.
- Boos, J.; Fang, J.; Heidinger, B.H.; Raptopoulos, V.; Brook, O.R. Dual energy CT angiography: Pros and cons of dual-energy metal artifact reduction algorithm in patients after endovascular aortic repair. Abdom. Radiol. 2017, 42, 749–758.
- Iezzi, R.; Carchesio, F.; Posa, A.; Colosimo, C.; Bonomo, L. Post-EVAR split-bolus CT angiography using dual-energy CT: All you need in a single scan! In EuroSafe Imaging; ESR: Vienna, Austria, 2017.
- Mehran, R.; Nikolsky, E. Contrast-induced nephropathy: Definition, epidemiology, and patients at risk. Kidney Int. 2006, 69, S11–S15.
- van der Molen, A.J.; Thomsen, H.S.; Morcos, S.K. Effect of iodinated contrast media on thyroid function in adults. Eur. Radiol. 2004, 14, 902–907.
- Katayama, H.; Yamaguchi, K.; Kozuka, T.; Takashima, T.; Seez, P.; Matsuura, K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990, 175, 621–628.
- Yamamoto, M.; Hayashida, K.; Mouillet, G.; Hovasse, T.; Chevalier, B.; Oguri, A.; Watanabe, Y.; Dubois-Randé, J.-L.; Morice, M.-C.; Lefèvre, T.; et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2013, 62, 869–877.
- Kane, G.C.; Doyle, B.J.; Lerman, A.; Barsness, G.W.; Best, P.J.; Rihal, C.S. Ultra-Low Contrast Volumes Reduce Rates of Contrast-Induced Nephropathy in Patients with Chronic Kidney Disease Undergoing Coronary Angiography. J. Am. Coll. Cardiol. 2008, 51, 89–90.
- McDonald, R.J.; McDonald, J.S.; Bida, J.P.; Carter, R.E.; Fleming, C.J.; Misra, S.; Williamson, E.E.; Kallmes, D.F.; Paltiel, H.J.; Gilligan, L.A.; et al. Intravenous contrast material-induced nephropathy: Causal or coincident phenomenon? Radiology 2013, 267, 106–118.
- Hu, D.; Yu, T.; Duan, X.; Peng, Y.; Zhai, R. Determination of the optimal energy level in spectral CT imaging for displaying abdominal vessels in pediatric patients. Eur. J. Radiol. 2014, 83, 589–594.
- Huda, W.; Scalzetti, E.M.; Levin, G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 2000, 217, 430–435.
- van Hamersvelt, R.W.; Eijsvoogel, N.G.; Mihl, C.; de Jong, P.A.; Schilham, A.M.R.; Buls, N.; Das, M.; Leiner, T.; Willemink, M.J. Contrast agent concentration optimization in CTA using low tube voltage and dual-energy CT in multiple vendors: A phantom study. Int. J. Cardiovasc. Imaging 2018, 34, 1265–1275.
- Carrascosa, P.; Leipsic, J.A.; Capunay, C.; Deviggiano, A.; Vallejos, J.; Goldsmit, A.; Rodriguez-Granillo, G.A. Monochromatic image reconstruction by dual energy imaging allows half iodine load computed tomography coronary angiography. Eur. J. Radiol. 2015, 84, 1915–1920.
- Godoy, M.C.; Heller, S.L.; Naidich, D.P.; Assadourian, B.; Leidecker, C.; Schmidt, B.; Vlahos, I. Dual-energy MDCT: Comparison of pulmonary artery enhancement on dedicated CT pulmonary angiography, routine and low contrast volume studies. Eur. J. Radiol. 2011, 79, e11–e17.
- Nijhof, W.; Baltussen, E.; Kant, I.; Jager, G.; Slump, C.; Rutten, M. Low-dose CT angiography of the abdominal aorta and reduced contrast medium volume: Assessment of image quality and radiation dose. Clin. Radiol. 2016, 71, 64–73.
- Dubourg, B.; Caudron, J.; Lestrat, J.-P.; Bubenheim, M.; Lefebvre, V.; Godin, M.; Tron, C.; Eltchaninoff, H.; Bauer, F.; Dacher, J.-N. Single-source dual-energy CT angiography with reduced iodine load in patients referred for aortoiliofemoral evaluation before transcatheter aortic valve implantation: Impact on image quality and radiation dose. Eur. Radiol. 2014, 24, 2659–2668.
- Martin, S.S.; Albrecht, M.H.; Wichmann, J.L.; Hüsers, K.; Scholtz, J.-E.; Booz, C.; Bodelle, B.; Bauer, R.W.; Metzger, S.C.; Vogl, T.J.; et al. Value of a noise-optimized virtual monoenergetic reconstruction technique in dual-energy CT for planning of transcatheter aortic valve replacement. Eur. Radiol. 2017, 27, 705–714.
- Maturen, K.E.; Kaza, R.K.; Liu, P.S.; Quint, L.E.; Khalatbari, S.H.; Platt, J.F. “Sweet spot” for endoleak detection: Optimizing contrast to noise using low kev reconstructions from fast-switch kVp dual-energy CT. J. Comput. Assist. Tomogr. 2012, 36, 83–87.
- Martin, S.S.; Wichmann, J.L.; Weyer, H.; Scholtz, J.-E.; Leithner, D.; Spandorfer, A.; Bodelle, B.; Jacobi, V.; Vogl, T.J.; Albrecht, M.H. Endoleaks after endovascular aortic aneurysm repair: Improved detection with noise-optimized virtual monoenergetic dual-energy CT. Eur. J. Radiol. 2017, 94, 125–132.
- Skawran, S.; Angst, F.; Blüthgen, C.; Eberhard, M.; Kälin, P.; Kobe, A.; Nagy, D.; Szucs-Farkas, Z.; Alkadhi, H.; Euler, A. Dual-Energy Low-keV or Single-Energy Low-kV CT for Endoleak Detection? Investig. Radiol. 2020, 55, 45–52.
- Charalambous, S.; Perisinakis, K.; Kontopodis, N.; Papadakis, A.E.; Maris, T.G.; Ioannou, C.V.; Karantanas, A.; Tsetis, D. Dual-energy CT angiography in imaging surveillance of endovascular aneurysm repair—Preliminary study results. Eur. J. Radiol. 2022, 148, 110165.
More
