The prevalence of childhood obesity and chronic kidney disease (CKD) is steadily increasing worldwide, reaching epidemic proportions. While the impact of obesity in children with CKD is less pronounced than in adults, recent studies suggest a similar trend in the child population. This is likely due to the significant association between obesity and the two leading causes of end-stage renal disease (ESRD): diabetes mellitus (DM) and hypertension. Obesity is a complex, systemic disease that reflects interactions between environmental and genetic factors. A key mechanism of kidney damage is related to metabolic syndrome and insulin resistance. Therefore, we can speculate about an adipose tissue–kidney axis in which neurohormonal and immunological mechanisms exacerbate complications resulting from obesity. Adipose tissue, now recognized as an endocrine organ, secretes cytokines called adipokines that may induce adaptive or maladaptive responses in renal cells, leading to kidney fibrosis. The impact of obesity on kidney transplant-related outcomes for both donors and recipients is also significant, making stringent preventive measures critical in the pre- and post-transplant phases. The challenge lies in identifying renal involvement as early as possible, as it is often completely asymptomatic and not detectable through common markers of kidney function. Ongoing research into innovative technologies, such as proteomics and metabolomics, aims to identify new biomarkers and is constantly evolving. Many aspects of pediatric disease progression in the population of children with obesity still require clarification. However, the latest scientific evidence in the field of nephrology offers glimpses into various new perspectives, such as genetic factors, comorbidities, and novel biomarkers. Investigating these aspects early could potentially improve the prognosis of these young patients through new diagnostic and therapeutic strategies.
- glomerular filtration rate (GFR)
- chronic kidney disease (CKD)
- biomarkers
- body mass index (BMI)
- glomerulopathy
1. Childhood Obesity Definition and BMI Related CKD Progression
2. Impact of Obesity on Kidney Outcomes in Children
3. Hereditary Factors of Obesity
- I.
-
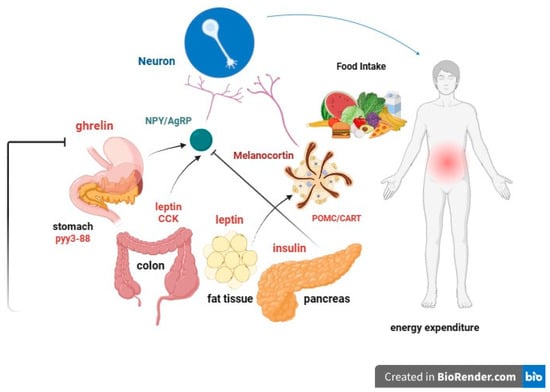
leptin: Produced by adipose cells in the placenta and, to a lesser extent, in the intestine. The Ob (Obese) or Lep (Leptin) gene codes for leptin, which signals to the brain, regarding the levels of stored fat in the body. Leptin-deficient mice (Ob mice) show hyperphagia, insulin resistance, hyperinsulinemia, and infertility. By increasing adiposity, resistance to the action of leptin occurs. Although obesity due to leptin deficiency has been studied, most people with obesity do not have any abnormalities in the leptin gene. This suggests that obesity may be caused by either leptin deficiency or genetic defects in the leptin receptor itself [49,50][36][37].
- II.
-
Prohormone convertase 1/3 (PC1/3): a congenital deficiency of the PCSK1 gene, responsible for proprotein convertase 1/3, can lead to a severe multihormonal disorder characterized by early-onset obesity [51][38].
- III.
-
Congenital deficiency of the melanocortin-4 receptor (MC4R): a congenital deficiency of MC4R is associated with early-onset obesity and above-average height [52,53,54][39][40][41].
- IV.
-
Proopiomelanocortin (POMC) or melanocyte-stimulating hormone (MSH): It transmits the appetite-suppressing effect of leptin through MC4R. Mutations in POMC gene can also lead to early-onset obesity due to severe hyperphagia. ACTH (AdrenoCorticoTropic Hormone) is produced by POMC in the hypothalamus, as is alpha-MSH, a key factor in reducing food intake [55,56][42][43].
- V.
-
Guanine nucleotide-binding protein G, alpha stimulant (GNAS) gene mutations: these are associated with Albright’s hereditary osteodystrophy (i.e., type 1 pseudohypoparathyroidism), and characterized by early-onset obesity, along with other features such as developmental delay, short stature, brachydactyly, subcutaneous ossifications, pseudohypoparathyroidism (hypocalcemia, resistance to parathormone), and resistance to thyrotropin (elevated thyroid stimulating hormone with normal or low-free thyroxine) [57][44].
4. Correlation between Low Birth Weight, Obesity, and CKD
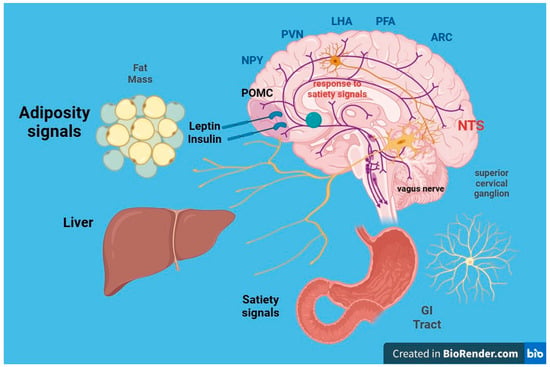
5. Neurohormonal, Metabolic and Immunological Effects of Obesity on Kidney Function
5.1. Insulin Resistance
5.2. Leptin
5.3. Adiponectin
5.4. Other Adipokines
6. Hypertension and Obesity in Children
Hypertension associated with obesity is a complex multifactorial disease, including activation of the RAAS, impaired vascular function, and increased sympathetic nervous system (SNS) activity [105][92]. While a well-established relationship exists between obesity and hypertension in adults, the prevalence of hypertension and pre-hypertension in the pediatric setting is significantly higher among obese children and adolescents [106,107][93][94]. The kidney is both a cause and victim of hypertension [108][95] because, on the one hand, the presence of reduced insulin sensitivity, dysglycemia, and dyslipidemia (both precursors of hypertension) can lead to renal damage, and on the other hand, functional and structural alterations in the nephrons can contribute to increased BP [109][96]. Hypertension is not only a risk factor for the progression of renal disease in the pediatric population with CKD [110][97], but it is also a significant contributor to renal dysfunction in obese patients, although it is not the sole hemodynamic cause [30][14]. Studies have shed light on the profound impact of adiposity on BP levels in the pediatric population. For instance, research by Tu et al. [111][98] revealed a four-fold increase in BP levels across all age groups in small patients with a BMI > 85th percentile. Similarly, Sorof et al. [112][99] demonstrated a progressive increase in hypertension prevalence in school-aged children as BMI exceeded the 95th percentile. Furthermore, an Italian study showed that prehypertensive children exhibited reduced GFR and increased proteinuria compared with normotensive children [113][100]. High-normal BP levels in the pre-hypertension group correlated with low-normal renal function, characterized by decreased GFR and increased proteinuria levels, albeit still within accepted normal ranges. Notably, left ventricular hypertrophy is more common in children with stage 2–4 CKD and with masked or confirmed hypertension [114][101], underlying the importance of identifying hypertension in these cases. Masked hypertension has emerged as a particularly strong independent predictor of left ventricular hypertrophy in the pediatric population, likely attributed to the hyperactivation of the RAAS [115][102]. The correlation between arterial hypertension and obesity in children involves several factors. These include insulin resistance, either alone or combined with hyperleptinemia, which activates the SNS, leading to vasoconstriction, reduced renal blood flow, and subsequent activation of RAAS and water and sodium retention [116][103]. Elevated leptin levels also contribute to increased BP and SNS activation. In the pediatric population, proinflammatory cytokines, oxidative stress pathways, sleep apnea syndrome, or poor sleep quality can further exacerbate arterial stiffness and endothelial dysfunction [117][104], increasing the risk of hypertension in obese children [118][105]. Moreover, low serum levels of 1,25-dihydroxyvitamin D (1,25-(OH)2D3) have been associated with metabolic syndrome and hypertension in Caucasian children and adolescents [119][106]. Hyperuricemia, which can result from a high fructose diet and increased uric acid production by adipose tissue in obese individuals, also represents a potential correlation mechanism between hypertension and obesity [120][107]. Studies in Moscow have shown hyperuricemia (>8.0 mg/dL) in a higher percentage of children with hypertension compared with those with normal BP [121][108]. Large-scale epidemiological studies are needed to further confirm these findings.7. Renal Biomarkers in Obese Children
CKD and renal injury due to obesity are frequently asymptomatic in the early stages and difficult to recognize, especially in the pediatric population, where individuals often remain underdiagnosed for several years. Therefore, reliable biomarkers are needed to prevent potentially very serious renal complications and dramatically improve the overall management of this disease. Traditional renal biomarkers include serum creatinine (sCr), blood urea nitrogen (BUN), urinary albumin/protein, and volume excretion values. However, sCr and BUN have limitations in distinguishing renal injury from hemodynamic changes, especially in acute kidney injury. Furthermore, their levels may not rapidly change in response to damage due to the presence of a functional reserve mechanism in nephrons. This “reservoir” consists of other nephrons that can increase their own function in response to injury. As a result, sCr and BUN levels often remain within the “normal range” until substantial injury has occurred, potentially leading to irreversible loss of a significant number of nephrons. Among the many equations available for estimating GFR, it has been seen that FAS age (height-independent full-age spectrum equation), FAS height (height-dependent full-age spectrum equation), and LMR18 (adjusted-creatinine revised Lund-Malmö equation) are the preferred serum creatinine-based formulas in children with overweight or obesity [122][109]. While measurements of GFR using methods such as sCr or iohexol clearance provide important insights into renal function, they may not closely parallel the extent of the renal lesion. In recent years, several studies have been conducted with the aim of identifying new biomarkers for early detection of kidney injury, although most of these are used exclusively in research settings and need further validation. Some of these (Table 1) include urinary glutamyl aminopeptidase (GluAp), urinary podocalyxin (PCX), urinary kidney injury molecule-1 (KIM-1), alpha-1-acid glycoprotein (AGP), urinary N-acetyl-beta-D-glucosaminidase (NAG), and urinary neutrophil gelatinase-associated lipocalin (NGAL) [123,124][110][111]. Overexpression of these biomarkers was evidenced in the obese pediatric population [125,126][112][113]. Thus, at present, there are very few human studies concerning biomarkers of early kidney damage secondary to obesity, particularly in pediatric populations. In obese children and adolescents, diagnostic screening strategies could be considered in order to identify any early functional and/or structural kidney injury and consequently reduce the likelihood of potential complications, improving their prognosis. However, currently, there is insufficient evidence to recommend screening for kidney complications in non-diabetic and non-hypertensive children and adolescents with obesity [127][114]. Cystatin C is another marker used for estimating GFR and is independent of muscle mass in children [128,129][115][116]. Although some studies, such as those by Miliku et al., suggest that both eGFR derived from creatinine (eGFRcreat) and cystatin C (eGFRcystC) blood concentrations can be influenced by factors such as BMI and body surface area, it is worth noting that eGFRcreat is more strongly affected by lean mass percentage and fat mass percentage [130][117]. Urinary cystatin has been found to be useful in detecting early kidney injury due to obesity in children [124][111]. An alternative biomarker for estimating renal function is rescaled serum creatinine (SCr/Q), which accounts for sex, age, or height. SCr/Q and almost all GFR estimations correlated with obesity-related comorbidities (anthropometric and metabolic variables) in 600 children with overweight and obesity without overt kidney disease [131][118]. Nevertheless, the progression of renal dysfunction in obesity often starts with microalbuminuria, which can eventually progress to overt proteinuria. A Framingham study has shown that overweight and obese individuals were more likely to develop proteinuria when compared to healthy individuals [132][119]. One dramatic demonstration of the impact of obesity on renal function was observed in patients who underwent unilateral nephrectomy. Among obese nephrotic patients, 92% developed proteinuria and impaired renal function over a 10-year follow-up period, whereas only 12% of non-obese subjects experienced such complications [133][120]. As mentioned previously, microalbuminuria functions as both an early marker of CKD and a marker of renal damage in non-diabetic patients. Csernus et al., for instance, showed that elevated levels of albuminuria and β2-microglobulinuria in obese children, compared with children with a normal body weight, suggest early renal glomerular and tubular damage, resulting from obesity [134][121]. Ferris et al. have also highlighted that microalbuminuria is strongly correlated with the severity of obesity in adults [135][122]. The use of multiple markers in nephrology will be useful as they can provide diverse information, including insights into the site of the lesion, the presence of inflammation, and any association with systemic diseases. Alpha-1-acid glycoprotein (AGP) is an acute-phase protein that has both pro- and anti-inflammatory actions. Medyńska et al. [136][123] have observed that α1-AGP increased before the onset of albuminuria, suggesting that it could be a biomarker of early glomerular damage in obese children. Lipocalin associated with neutrophilic gelatinase (NGAL) is one of the intriguing biomarkers in this context. NGAL is a 25 kDa protein associated with neutrophilic gelatinase, and it is known to be released by renal tubular cells damaged in acute kidney injury even before a decrease in GFR occurs [137][124]. Studies have identified NGAL concentrations in serum and urine as independent predictors of CKD progression [138][125] in patients with moderate renal disease, and it has been noted that its urinary concentrations may predict the development of CKD in obese adolescents with normal or reduced GFR [139][126]. Furthermore, NGAL is currently employed as an early biomarker for diabetic nephropathy [140][127]. Notably, in a study by Goknar et al., urine NGAL concentrations did not show significant differences between obese children and healthy controls [126][113]. However, Şen et al. observed that obese children with insulin resistance had elevated urinary levels of NGAL [141][128]. Thus, these investigations showed mixed results regarding the possible role of NGAL as a biomarker of early kidney damage due to obesity.References
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and development. Vital Health Stat. 2002, 11, 1–190.
- Kelly, A.S.; Barlow, S.E.; Rao, G.; Inge, T.H.; Hayman, L.L.; Steinberger, J.; Urbina, E.M.; Ewing, L.J.; Daniels, S.R. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the American Heart Association. Circulation 2013, 128, 1689–1712.
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L.J. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33.
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Culleton, B.; Wilson, P.W.; Levy, D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004, 291, 844–850.
- Russo, D.; Morrone, L.F.; Errichiello, C.; De Gregorio, M.G.; Imbriaco, M.; Battaglia, Y.; Russo, L.; Andreucci, M.; Di Iorio, B.R. Impact of BMI on cardiovascular events, renal function, and coronary artery calcification. Blood Purif. 2014, 38, 1–6.
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235.
- Postorino, M.; Marino, C.; Tripepi, G.; Zoccali, C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J. Am. Coll. Cardiol. 2009, 53, 1265–1272.
- Hsu, C.Y.; McCulloch, C.E.; Iribarren, C.; Darbinian, J.; Go, A.S. Body mass index and risk for end-stage renal disease. Ann. Intern. Med. 2006, 144, 21–28.
- Maring, B.; Greenspan, L.C.; Chandra, M.; Daniels, S.R.; Sinaiko, A.; Prineas, R.J.; Parker, E.D.; Adams, K.F.; Daley, M.F.; Sherwood, N.E.; et al. Comparing US paediatric and adult weight classification at the transition from late teenage to young adulthood. Pediatr. Obes. 2015, 10, 371–379.
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P., Jr.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683.
- Gepstein, V.; Weiss, R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 568.
- Stern-Zimmer, M.; Calderon-Margalit, R.; Skorecki, K.; Vivante, A. Childhood risk factors for adulthood chronic kidney disease. Pediatr. Nephrol. 2021, 36, 1387–1396.
- Hernandez, G.T.; Nasri, H. World Kidney Day 2014: Increasing awareness of chronic kidney disease and aging. J. Ren. Inj. Prev. 2014, 3, 3–4.
- Wahba, I.M.; Mak, R.H. Obesity and Obesity-Initiated Metabolic Syndrome: Mechanistic Links to Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562.
- Batsis, J.A.; Romero-Corral, A.; Collazo-Clavell, M.L.; Sarr, M.G.; Somers, V.K.; Lopez-Jimenez, F. Effect of bariatric surgery on the metabolic syndrome: A population-based, long-term controlled study. Mayo Clin. Proc. 2008, 83, 897–907.
- Sun, J.; Wang, C.; Zhao, M.; Lee, P.M.Y.; Xi, B.; Yu, Y.; Li, J. Childhood diabetes mellitus and early-onset kidney diseases later in life: A nationwide population-based matched cohort study. BMC Med. 2022, 20, 428.
- Vivante, A.; Golan, E.; Tzur, D.; Leiba, A.; Tirosh, A.; Skorecki, K.; Calderon-Margalit, R. Body Mass Index in 1.2 Million Adolescents and Risk for End-Stage Renal Disease. Arch. Intern. Med. 2012, 172, 1644–1650.
- Harambat, J.; van Stralen, K.J.; Kim, J.J.; Tizard, E.J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 2012, 27, 363–373.
- Jadresic, L.; Silverwood, R.J.; Kinra, S.; Nitsch, D. Can childhood obesity influence later chronic kidney disease? Pediatr. Nephrol. 2019, 34, 2457–2477.
- Pourghazi, F.; Mohammadi, S.; Eslami, M.; Zoshk, M.Y.; Asadi, S.; Ejtahed, H.S.; Qorbani, M. Association between Childhood Obesity and Later Life Kidney Disorders: A Systematic Review. J. Ren. Nutr. 2023, 33, 520–528.
- Lalan, S.; Jiang, S.; Ng, D.K.; Kupferman, F.; Warady, B.A.; Furth, S.; Mitsnefes, M.M. Cardiometabolic Risk Factors, Metabolic Syndrome, and Chronic Kidney Disease Progression in Children. J. Pediatr. 2018, 202, 163–170.
- Correia-Costa, L.; Azevedo, A.; Caldas Afonso, A. Childhood Obesity and Impact on the Kidney. Nephron 2019, 143, 8–11.
- Silverwood, R.J.; Pierce, M.; Hardy, R.; Thomas, C.; Ferro, C.; Savage, C.; Sattar, N.; Kuh, D.; Nitsch, D. Early-life overweight trajectory and CKD in the 1946 British birth cohort study. Am. J. Kidney Dis. 2013, 62, 276–284.
- Silverwood, R.J.; Pierce, M.; Thomas, C.; Hardy, R.; Ferro, C.; Sattar, N.; Whincup, P.; Savage, C.; Kuh, D.; Nitsch, D. Association between younger age when first overweight and increased risk for CKD. J. Am. Soc. Nephrol. 2013, 24, 813–821.
- Filler, G.; Reimão, S.M.; Kathiravelu, A.; Grimmer, J.; Feber, J.; Drukker, A. Pediatric nephrology patients are overweight: 20 years’ experience in a single Canadian tertiary pediatric nephrology clinic. Int. Urol. Nephrol. 2007, 39, 1235–1240.
- Bonthuis, M.; van Stralen, K.J.; Verrina, E.; Groothoff, J.W.; Alonso Melgar, Á.; Edefonti, A.; Fischbach, M.; Mendes, P.; Molchanova, E.A.; Paripović, D.; et al. Underweight, overweight and obesity in paediatric dialysis and renal transplant patients. Nephrol. Dial. Transplant. 2013, 28, iv195–iv204.
- Wong, C.S.; Gipson, D.S.; Gillen, D.L.; Emerson, S.; Koepsell, T.; Sherrard, D.J.; Watkins, S.L.; Stehman-Breen, C. Anthropometric measures and risk of death in children with end-stage renal disease. Am. J. Kidney Dis. 2000, 36, 811–819.
- Berthoud, H.R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008, 59, 55–92.
- Loos, R.J. Genetic determinants of common obesity and their value in prediction. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 211–226.
- Aucella, F.; Gesuete, A.; Battaglia, Y. A “nephrological” approach to physical activity. Kidney Blood Press. Res. 2014, 39, 189–196.
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894.
- Dina, C.; Meyre, D.; Gallina, S.; Durand, E.; Körner, A.; Jacobson, P.; Carlsson, L.M.; Kiess, W.; Vatin, V.; Lecoeur, C.; et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 2007, 39, 724–726.
- Claussnitzer, M.; Dankel, S.N.; Kim, K.H.; Quon, G.; Meuleman, W.; Haugen, C.; Glunk, V.; Sousa, I.S.; Beaudry, J.L.; Puviindran, V.; et al. FTO Obesity Variant Circuitry and Adipocyte Browning in Humans. N. Engl. J. Med. 2015, 373, 895–907.
- Czajkowski, P.; Adamska-Patruno, E.; Bauer, W.; Fiedorczuk, J.; Krasowska, U.; Moroz, M.; Gorska, M.; Kretowski, A. The Impact of FTO Genetic Variants on Obesity and Its Metabolic Consequences is Dependent on Daily Macronutrient Intake. Nutrients 2020, 12, 3255.
- Gaulton, K.J. Mechanisms of Type 2 Diabetes Risk Loci. Curr. Diabetes Rep. 2017, 17, 72.
- Trasande, L.; Cronk, C.; Durkin, M.; Weiss, M.; Schoeller, D.; Gall, E.; Hewitt, J.; Carrel, A.; Landrigan, P.; Gillman, M. Environment and obesity in the National Children’s Study. Cienc. Saude Coletiva 2010, 15, 195–210.
- Dardeno, T.A.; Chou, S.H.; Moon, H.S.; Chamberland, J.P.; Fiorenza, C.G.; Mantzoros, C.S. Leptin in human physiology and therapeutics. Front. Neuroendocrinol. 2010, 31, 377–393.
- Stijnen, P.; Tuand, K.; Varga, T.V.; Franks, P.W.; Aertgeerts, B.; Creemers, J.W. The association of common variants in PCSK1 with obesity: A HuGE review and meta-analysis. Am. J. Epidemiol. 2014, 180, 1051–1065.
- Lubrano-Berthelier, C.; Le Stunff, C.; Bougnères, P.; Vaisse, C. A homozygous null mutation delineates the role of the melanocortin-4 receptor in humans. J. Clin. Endocrinol. Metab. 2004, 89, 2028–2032.
- Savastano, D.M.; Tanofsky-Kraff, M.; Han, J.C.; Ning, C.; Sorg, R.A.; Roza, C.A.; Wolkoff, L.E.; Anandalingam, K.; Jefferson-George, K.S.; Figueroa, R.E.; et al. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. Am. J. Clin. Nutr. 2009, 90, 912–920.
- Farooqi, I.S.; Yeo, G.S.; Keogh, J.M.; Aminian, S.; Jebb, S.A.; Butler, G.; Cheetham, T.; O’Rahilly, S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000, 106, 271–279.
- Krude, H.; Biebermann, H.; Schnabel, D.; Tansek, M.Z.; Theunissen, P.; Mullis, P.E.; Grüters, A. Obesity due to proopiomelanocortin deficiency: Three new cases and treatment trials with thyroid hormone and ACTH4-10. J. Clin. Endocrinol. Metab. 2003, 88, 4633–4640.
- Cignarelli, M.; Lamacchia, O. Obesity and kidney disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 757–762.
- Mendes de Oliveira, E.; Keogh, J.M.; Talbot, F.; Henning, E.; Ahmed, R.; Perdikari, A.; Bounds, R.; Wasiluk, N.; Ayinampudi, V.; Barroso, I.; et al. Obesity-Associated GNAS Mutations and the Melanocortin Pathway. N. Engl. J. Med. 2021, 385, 1581–1592.
- Hoy, W.E.; Rees, M.; Kile, E.; Mathews, J.D.; Wang, Z. A new dimension to the Barker hypothesis: Low birthweight and susceptibility to renal disease. Kidney Int. 1999, 56, 1072–1077.
- Phillips, D.I.; Jones, A.; Goulden, P.A. Birth weight, stress, and the metabolic syndrome in adult life. Ann. N. Y. Acad. Sci. 2006, 1083, 28–36.
- Abitbol, C.L.; Chandar, J.; Rodríguez, M.M.; Berho, M.; Seeherunvong, W.; Freundlich, M.; Zilleruelo, G. Obesity and preterm birth: Additive risks in the progression of kidney disease in children. Pediatr. Nephrol. 2009, 24, 1363–1370.
- Greenbaum, L.A.; Muñoz, A.; Schneider, M.F.; Kaskel, F.J.; Askenazi, D.J.; Jenkins, R.; Hotchkiss, H.; Moxey-Mims, M.; Furth, S.L.; Warady, B.A. The association between abnormal birth history and growth in children with CKD. Clin. J. Am. Soc. Nephrol. 2011, 6, 14–21.
- Grillo, M.A.; Mariani, G.; Ferraris, J.R. Prematurity and Low Birth Weight in Neonates as a Risk Factor for Obesity, Hypertension, and Chronic Kidney Disease in Pediatric and Adult Age. Front. Med. 2021, 8, 769734.
- Chen, J.; Muntner, P.; Hamm, L.L.; Jones, D.W.; Batuman, V.; Fonseca, V.; Whelton, P.K.; He, J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 2004, 140, 167–174.
- Blüher, M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43.
- González, E.; Gutiérrez, E.; Morales, E.; Hernández, E.; Andres, A.; Bello, I.; Díaz-González, R.; Leiva, O.; Praga, M. Factors influencing the progression of renal damage in patients with unilateral renal agenesis and remnant kidney. Kidney Int. 2005, 68, 263–270.
- Marcantoni, C.; Ma, L.J.; Federspiel, C.; Fogo, A.B. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002, 62, 172–180.
- Zhao, H.L.; Sui, Y.; Guan, J.; He, L.; Zhu, X.; Fan, R.R.; Xu, G.; Kong, A.P.; Ho, C.S.; Lai, F.M.; et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int. 2008, 74, 467–477.
- Roubicek, T.; Bartlova, M.; Krajickova, J.; Haluzikova, D.; Mraz, M.; Lacinova, Z.; Kudla, M.; Teplan, V.; Haluzik, M. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition 2009, 25, 762–768.
- Martinez Cantarin, M.P.; Whitaker-Menezes, D.; Lin, Z.; Falkner, B. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol. Dial. Transplant. 2017, 32, 943–951.
- Ambarkar, M.; Pemmaraju, S.V.; Gouroju, S.; Manohar, S.M.; Bitla, A.R.; Yajamanam, N.; Vishnubhotla, S. Adipokines and their Relation to Endothelial Dysfunction in Patients with Chronic Kidney Disease. J. Clin. Diagn. Res. 2016, 10, BC04–BC08.
- Xiang, D.M.; Song, X.Z.; Zhou, Z.M.; Liu, Y.; Dai, X.Y.; Huang, X.L.; Hou, F.F.; Zhou, Q.G. Chronic kidney disease promotes chronic inflammation in visceral white adipose tissue. Am. J. Physiol. Ren. Physiol. 2017, 312, F689–F701.
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213.
- Martos-Rus, C.; Katz-Greenberg, G.; Lin, Z.; Serrano, E.; Whitaker-Menezes, D.; Domingo-Vidal, M.; Roche, M.; Ramaswamy, K.; Hooper, D.C.; Falkner, B.; et al. Macrophage and adipocyte interaction as a source of inflammation in kidney disease. Sci. Rep. 2021, 11, 2974.
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477.
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998.
- Chun, T.H.; Hotary, K.B.; Sabeh, F.; Saltiel, A.R.; Allen, E.D.; Weiss, S.J. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 2006, 125, 577–591.
- Xiao, Y.; Liu, D.; Cline, M.A.; Gilbert, E.R. Chronic stress, epigenetics, and adipose tissue metabolism in the obese state. Nutr. Metab. 2020, 17, 88.
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules 2020, 25, 5218.
- Shankar, A.; Syamala, S.; Xiao, J.; Muntner, P. Relationship between Plasma Leptin Level and Chronic Kidney Disease. Int. J. Nephrol. 2012, 2012, 269532.
- Blüher, M. Adipokines—Removing road blocks to obesity and diabetes therapy. Mol. Metab. 2014, 3, 230–240.
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470.
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223.
- Lee, B.T.; Ahmed, F.A.; Hamm, L.L.; Teran, F.J.; Chen, C.-S.; Liu, Y.; Shah, K.; Rifai, N.; Batuman, V.; Simon, E.E.; et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol. 2015, 16, 77.
- Tesauro, M.; Mascali, A.; Franzese, O.; Cipriani, S.; Cardillo, C.; Di Daniele, N. Chronic kidney disease, obesity, and hypertension: The role of leptin and adiponectin. Int. J. Hypertens. 2012, 2012, 943605.
- Nguyen, S.; McCulloch, C.; Brakeman, P.; Portale, A.; Hsu, C.Y. Being overweight modifies the association between cardiovascular risk factors and microalbuminuria in adolescents. Pediatrics 2008, 121, 37–45.
- Nielsen, R.; Christensen, E.I.; Birn, H. Megalin and cubilin in proximal tubule protein reabsorption: From experimental models to human disease. Kidney Int. 2016, 89, 58–67.
- Kazmi, A.; Sattar, A.; Hashim, R.; Khan, S.P.; Younus, M.; Khan, F.A. Serum leptin values in the healthy obese and non-obese subjects of Rawalpindi. J. Pak. Med. Assoc. 2013, 63, 245–248.
- Mao, S.; Fang, L.; Liu, F.; Jiang, S.; Wu, L.; Zhang, J. Leptin and chronic kidney diseases. J. Recept. Signal Transduct. Res. 2018, 38, 89–94.
- Wolf, G.; Ziyadeh, F.N. Leptin and renal fibrosis. Contrib. Nephrol. 2006, 151, 175–183.
- Lee, M.P.; Orlov, D.; Sweeney, G. Leptin induces rat glomerular mesangial cell hypertrophy, but does not regulate hyperplasia or apoptosis. Int. J. Obes. 2005, 29, 1395–1401.
- Briffa, J.F.; McAinch, A.J.; Poronnik, P.; Hryciw, D.H. Adipokines as a link between obesity and chronic kidney disease. Am. J. Physiol. Physiol. 2013, 305, F1629–F1636.
- Noor, S.; Alam, F.; Fatima, S.S.; Khan, M.; Rehman, R. Role of Leptin and dyslipidemia in chronic kidney disease. Pak. J. Pharm. Sci. 2018, 31, 893–897.
- Liu, B.; Qiao, J.; Hu, J.; Fan, M.; Zhao, Y.; Su, H.; Wang, Z.; Yu, Q.; Ma, Q.; Li, Y.; et al. Leptin promotes endothelial dysfunction in chronic kidney disease by modulating the MTA1-mediated WNT/β-catenin pathway. Mol. Cell. Biochem. 2020, 473, 155–166.
- Alix, P.M.; Guebre-Egziabher, F.; Soulage, C.O. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie 2014, 105, 12–21.
- Esfahani, M.; Movahedian, A.; Baranchi, M.; Goodarzi, M.T. Adiponectin: An adipokine with protective features against metabolic syndrome. Iran. J. Basic Med. Sci. 2015, 18, 430–442.
- Zhu, Q.; Scherer, P.E. Immunologic and endocrine functions of adipose tissue: Implications for kidney disease. Nat. Rev. Nephrol. 2018, 14, 105–120.
- Ohashi, K.; Iwatani, H.; Kihara, S.; Nakagawa, Y.; Komura, N.; Fujita, K.; Maeda, N.; Nishida, M.; Katsube, F.; Shimomura, I.; et al. Exacerbation of Albuminuria and Renal Fibrosis in Subtotal Renal Ablation Model of Adiponectin-Knockout Mice. Arter. Thromb. Vasc. Biol. 2007, 27, 1910–1917.
- Sawaguchi, T.; Nakajima, T.; Haruyama, A.; Hasegawa, T.; Shibasaki, I.; Nakajima, T.; Kaneda, H.; Arikawa, T.; Obi, S.; Sakuma, M.; et al. Association of serum leptin and adiponectin concentrations with echocardiographic parameters and pathophysiological states in patients with cardiovascular disease receiving cardiovascular surgery. PLoS ONE 2019, 14, e0225008.
- Jing, Y.; Jin, S.; Qiao, R.; Fang, J. Association of adipocytokines with obesity and insulin resistance in Korean-Chinese and Han nationality pupils of Yanbian area. Wei Sheng Yan Jiu 2015, 44, 581–585.
- Song, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Oh, K.H.; Ahn, C.; Kim, S.W.; Bae, E.H. High serum adiponectin as a biomarker of renal dysfunction: Results from the KNOW-CKD study. Sci. Rep. 2020, 10, 5598.
- Kuo, I.C.; Wu, P.H.; Lin, H.Y.; Niu, S.W.; Huang, J.C.; Hung, C.C.; Chiu, Y.W.; Chen, H.C. The association of adiponectin with metabolic syndrome and clinical outcome in patients with non-diabetic chronic kidney disease. PLoS ONE 2019, 14, e0220158.
- Pabalan, N.; Tiongco, R.E.; Pandac, J.K.; Paragas, N.A.; Lasta, S.L.; Gallego, N.; Jarjanazi, H.; Pineda-Cortel, M.R. Association and biomarker potential of elevated serum adiponectin with nephropathy among type 1 and type 2 diabetics: A meta-analysis. PLoS ONE 2018, 13, e0208905.
- Briones, A.M.; Cat, A.N.D.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes Produce Aldosterone through Calcineurin-Dependent Signaling Pathways. Hypertension 2012, 59, 1069–1078.
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.J.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471.
- Kotchen, T.A. Obesity-Related Hypertension: Epidemiology, Pathophysiology, and Clinical Management. Am. J. Hypertens. 2010, 23, 1170–1178.
- Falkner, B.; Gidding, S. Childhood Obesity and Blood Pressure. Hypertension 2011, 58, 754–755.
- Battaglia, Y.; Esposito, P.; Corrao, S.; Russo, L.; Balducci, A.; Storari, A.; Russo, D. Evaluation of Hypertension, Proteinuria, and Abnormalities of Body Weight in Italian Adolescents Participating in the World Kidney Days. Kidney Blood Press. Res. 2020, 45, 286–296.
- Bakris, G.L.; Ritz, E. The message for World Kidney Day 2009: Hypertension and kidney disease: A marriage that should be prevented. Clin. J. Am. Soc. Nephrol. 2009, 4, 517–519.
- El-Atat, F.A.; Stas, S.N.; McFarlane, S.I.; Sowers, J.R. The Relationship between Hyperinsulinemia, Hypertension and Progressive Renal Disease. J. Am. Soc. Nephrol. 2004, 15, 2816–2827.
- Staples, A.O.; Greenbaum, L.A.; Smith, J.M.; Gipson, D.S.; Filler, G.; Warady, B.A.; Martz, K.; Wong, C.S. Association between clinical risk factors and progression of chronic kidney disease in children. Clin. J. Am. Soc. Nephrol. 2010, 5, 2172–2179.
- Tu, W.; Eckert, G.J.; DiMeglio, L.A.; Yu, Z.; Jung, J.; Pratt, J.H. Intensified effect of adiposity on blood pressure in overweight and obese children. Hypertension 2011, 58, 818–824.
- Sorof, J.M.; Lai, D.; Turner, J.; Poffenbarger, T.; Portman, R.J. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004, 113 (3 Pt 1), 475–482.
- Lubrano, R.; Travasso, E.; Raggi, C.; Guido, G.; Masciangelo, R.; Elli, M. Blood pressure load, proteinuria and renal function in pre-hypertensive children. Pediatr. Nephrol. 2009, 24, 823–831.
- Mitsnefes, M.; Flynn, J.; Cohn, S.; Samuels, J.; Blydt-Hansen, T.; Saland, J.; Kimball, T.; Furth, S.; Warady, B. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J. Am. Soc. Nephrol. 2010, 21, 137–144.
- Shatat, I.F.; Flynn, J.T. Relationships between renin, aldosterone, and 24-hour ambulatory blood pressure in obese adolescents. Pediatr. Res. 2011, 69, 336–340.
- Flynn, J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr. Nephrol. 2013, 28, 1059–1066.
- Bucher, B.S.; Ferrarini, A.; Weber, N.; Bullo, M.; Bianchetti, M.G.; Simonetti, G.D. Primary hypertension in childhood. Curr. Hypertens. Rep. 2013, 15, 444–452.
- Archbold, K.H.; Vasquez, M.M.; Goodwin, J.L.; Quan, S.F. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: Report from the Tucson Children’s Assessment of Sleep Apnea Study. J. Pediatr. 2012, 161, 26–30.
- Pacifico, L.; Anania, C.; Osborn, J.F.; Ferraro, F.; Bonci, E.; Olivero, E.; Chiesa, C. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur. J. Endocrinol. 2011, 165, 603–611.
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315.
- Yanik, M.; Feig, D.I. Serum urate: A biomarker or treatment target in pediatric hypertension? Curr. Opin. Cardiol. 2013, 28, 433–438.
- van Dam, M.; Pottel, H.; Vreugdenhil, A.C.E. Creatinine-based GFR-estimating equations in children with overweight and obesity. Pediatr. Nephrol. 2022, 37, 2393–2403.
- Kim, S.S.; Song, S.H.; Kim, I.J.; Jeon, Y.K.; Kim, B.H.; Kwak, I.S.; Lee, E.K.; Kim, Y.K. Urinary Cystatin C and Tubular Proteinuria Predict Progression of Diabetic Nephropathy. Diabetes Care 2013, 36, 656–661.
- Ding, W.; Mak, R.H. Early markers of obesity-related renal injury in childhood. Pediatr. Nephrol. 2015, 30, 1–4.
- Nielsen, S.E.; Reinhard, H.; Zdunek, D.; Hess, G.; Gutiérrez, O.M.; Wolf, M.; Parving, H.H.; Jacobsen, P.K.; Rossing, P. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res. Clin. Pract. 2012, 97, 71–76.
- Goknar, N.; Oktem, F.; Ozgen, I.T.; Torun, E.; Kuçukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2015, 30, 139–144.
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88.
- Filler, G.; Bökenkamp, A.; Hofmann, W.; Le Bricon, T.; Martínez-Brú, C.; Grubb, A. Cystatin C as a marker of GFR--history, indications, and future research. Clin. Biochem. 2005, 38, 1–8.
- Bacchetta, J.; Cochat, P.; Rognant, N.; Ranchin, B.; Hadj-Aissa, A.; Dubourg, L. Which creatinine and cystatin C equations can be reliably used in children? Clin. J. Am. Soc. Nephrol. 2011, 6, 552–560.
- Miliku, K.; Bakker, H.; Dorresteijn, E.M.; Cransberg, K.; Franco, O.H.; Felix, J.F.; Jaddoe, V.W. Childhood Estimates of Glomerular Filtration Rate Based on Creatinine and Cystatin C: Importance of Body Composition. Am. J. Nephrol. 2017, 45, 320–326.
- van Dam, M.; Pottel, H.; Vreugdenhil, A.C.E. Relation between obesity-related comorbidities and kidney function estimation in children. Pediatr. Nephrol. 2023, 38, 1867–1876.
- Foster, M.C.; Hwang, S.J.; Larson, M.G.; Lichtman, J.H.; Parikh, N.I.; Vasan, R.S.; Levy, D.; Fox, C.S. Overweight, obesity, and the development of stage 3 CKD: The Framingham Heart Study. Am. J. Kidney Dis. 2008, 52, 39–48.
- Praga, M.; Hernández, E.; Herrero, J.C.; Morales, E.; Revilla, Y.; Díaz-González, R.; Rodicio, J.L. Influence of obesity on the appearance of proteinuria and renal insufficiency after unilateral nephrectomy. Kidney Int. 2000, 58, 2111–2118.
- Csernus, K.; Lanyi, E.; Erhardt, E.; Molnar, D. Effect of childhood obesity and obesity-related cardiovascular risk factors on glomerular and tubular protein excretion. Eur. J. Pediatr. 2005, 164, 44–49.
- Ferris, M.; Hogan, S.L.; Chin, H.; Shoham, D.A.; Gipson, D.S.; Gibson, K.; Yilmaz, S.; Falk, R.J.; Jennette, J.C. Obesity, albuminuria, and urinalysis findings in US young adults from the Add Health Wave III study. Clin. J. Am. Soc. Nephrol. 2007, 2, 1207–1214.
- Medyńska, A.; Chrzanowska, J.; Kościelska-Kasprzak, K.; Bartoszek, D.; Żabińska, M.; Zwolińska, D. Alpha-1 Acid Glycoprotein and Podocin mRNA as Novel Biomarkers for Early Glomerular Injury in Obese Children. J. Clin. Med. 2021, 10, 4129.
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543.
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344.
- Mackowiak-Lewandowicz, K.; Ostalska-Nowicka, D.; Zaorska, K.; Kaczmarek, E.; Zachwieja, J.; Witt, M.; Nowicki, M. Chronic kidney disease predictors in obese adolescents. Pediatr. Nephrol. 2022, 37, 2479–2488.
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press. Res. 2009, 32, 91–98.
- Şen, S.; Özalp Kızılay, D.; Taneli, F.; Özen, Ç.; Ertan, P.; Özunan, İ.; Yıldız, R.; Ersoy, B. Urinary NGAL is a Potential Biomarker for Early Renal Injury in Insulin Resistant Obese Non-diabetic Children. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 400–407.
