MicroRNAs (miRNAs) are noncoding small RNAs that regulate the protein expression of coding messenger RNAs. They are used as biomarkers to aid in diagnosing, prognosticating, and surveillance of diseases, especially solid cancers. MiR-193a was shown to be directly pathogenic in an experimental mouse model of focal segmental glomerulosclerosis (FSGS). Its specific binding and downregulation of Wilm’s tumor-1 (WT-1), a transcription factor regulating podocyte phenotype, is documented. Also, miR-193a is a regulator switch causing the transdifferentiation of glomerular parietal epithelial cells to a podocyte phenotype in in vitro study. Interaction between miR-193a and apolipoprotein 1 (APOL1) mRNA in glomeruli (filtration units of kidneys) is potentially involved in the pathogenesis of common glomerular diseases.
1. Introduction
Glomerular diseases, including primary and secondary causes like diabetes, are the most common cause of end-stage kidney disease. End-stage kidney disease (ESKD) is characterized by the loss of kidney function—a glomerular filtration rate <15 mL/min or less requiring dialysis or kidney transplantation. ESKD incidence has increased steadily worldwide
[1]. Most glomerular diseases involve podocyte injury with variable involvement of other components of glomeruli (
Figure 1); some start with podocyte injury, and others result in podocyte injury. Further, progress in understanding podocyte biology has been rapid, and newer players are increasingly identified. MicroRNAs (miRNAs) are involved in critical cellular processes in developing and maintaining the structure of resident kidney cells
[2] (
Figure 1) and are essential regulators of disease pathways. miR-193a is an example that was proven to directly cause FSGS in an experimental model
[3]. Following the initial publication, there has been an explosive interest in this molecule among researchers working on kidney diseases.
Figure 1. Schematic diagram showing a glomerular structure. (A) Glomerulus is a conglomeration of capillaries (Cap) starting from an afferent arteriole and ending in an efferent arteriole and residing in a Bowman’s capsule (BC). The outer wall of the Bowman’s capsule is lined by parietal epithelial cells (PECs), podocytes form the inner wall, and the filtrate passes through Bowman’s space (BC) to a tubule. Endothelial cells form the capillaries wrapped by podocytes (PD). Mesangial cells (MCs) and matrix remain in the center of capillaries composed of various cells. (B) A capillary is wrapped by podocytes. (C) Schematic representation of critical adherens junction proteins, which maintain the integrity of the slit diaphragm.
MicroRNAs (miRNAs) are noncoding small regulatory RNAs (18–22 nucleotides) that act as post-transcriptional repressors of target messenger RNAs (mRNAs). They were discovered in the 1990s
[4], and approximately 2600 microRNAs are observed to regulate 60% of human protein-coding genes
[5]. Like typical RNA, miRNAs are formed in the nucleus as primary miRNAs (pri-miRNA), a stem–loop structure containing two double-stranded RNAs. Pri-miRNA is cleaved into precursor miRNA (pre-miRNA), a stem–loop structure containing one double-stranded RNA, by an RNase III enzyme called Drosha. Pre-miRNA is exported to the cytoplasm, where it is cleaved into a double-stranded miRNA by another RNase III enzyme called Dicer, which gets incorporated into the RNA-induced silencing complex. Here, one strand of the double-stranded miRNA is degraded, making only one strand free to bind the target mRNA. The miRNAs are named miR-5p or miR-3p based on the original strand of pre-miRNA from which they are derived. The 5’ region of the miRNA (miR-5p pr miR-3p) binds to the 3’ untranslated region of the target mRNA to repress it
[6]. miRNAs are transported in body fluids inside exosomes. Exosomes are formed when multivesicular bodies inside cells (parts of endosomes)
[7] fuse with the plasma membrane and are shed
[8]. Exosomes contain a unique pattern of RNAs, DNAs, and miRNAs based on their cellular origin. Most cells in the human body can secrete exosomes, and it is apparent now that exosomes are a way of intercellular communication. Exosomes carrying miRNAs are used as biomarkers of various types of cancers, as specific tumor cells are observed to release exosomes containing unique profiles of miRNAs
[9]. Exosomes carrying individual miRNA profiles in the blood and urine are being investigated in glomerular diseases
[10].
One type of microRNA can regulate 100 different protein-coding genes, and different microRNAs can target a single protein-coding gene. They are crucial in biological pathways such as cell proliferation, differentiation, apoptosis, tumorigenesis, and fibrosis. miRNAs are involved in kidney development and diseases such as allograft rejection, acute kidney injury, and chronic kidney disease, including glomerulonephritis
[2]. Different types of miRNAs can have a contradictory effect on different cells, e.g., miR-21 has an antiapoptotic effect on tubular cells, whereas miR-193a is proapoptotic for podocytes. miR-30 and miR-146a are anti-inflammatory and antifibrotic, whereas miR92a and miR-193a are profibrotic. miR-193a increases cell proliferation and migration in renal cell carcinoma
[11], osteosarcoma, and pancreatic carcinoma
[12]; however, it has tumor-suppressive effects in other cancers, such as colorectal, breast, and gastric cancer
[13]. The upregulation of specific miRNAs and the downregulation of other specific miRNAs are associated with diseases and are determined by genetic and epigenetic changes
[12].
Using miRNAs as biomarkers to diagnose glomerular diseases and using miRNA-based therapeutics is a promising approach for treating glomerular diseases (
Figure 2). miRNAs can be measured in body fluids and tissues using quantitative polymerase chain reaction (PCR), next-generation sequencing, and microarray method
[14][76].
Figure 2.
Potential clinical applications of miR-193a in glomerular diseases.
2. MiR-193a as a Biomarker for Diagnosis and Prognosis of Glomerular Diseases
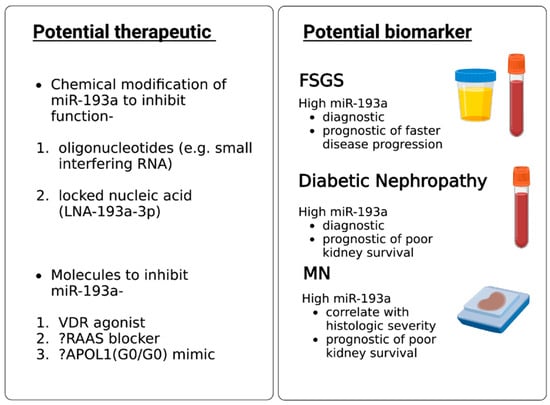
FSGS is diagnosed based on kidney biopsy, an invasive procedure. No single factor is known to cause primary FSGS; therefore, no biomarker has been employed to predict primary FSGS satisfactorily. The prognosis of FSGS is still assessed by response to glucocorticoids, which are associated with intolerable side effects. There is no reliable biomarker to prognosticate FSGS yet. Urinary exosomal miR-193a helps differentiate FSGS from minimal change disease in children
[15][74]. Higher urinary miR-193a predicted faster progression of FSGS in children
[16][64]. Higher levels of miR-193a, combined with the downregulation of WT-1 and podocalyxin, predict poor kidney survival in MN patients
[17][27]. Similarly, an increased plasma miR-193a level predicted diabetic nephropathy in patients with diabetes and was associated with shortened kidney survival
[18][63]. In a nutshell, miR-193a in urine and plasma seems to be a potentially helpful biomarker for FSGS diagnosis in children with nephrotic syndrome, for diabetic nephropathy diagnosis in patients with diabetes, and as a predictor of poor prognosis in FSGS, DN, and MN if validated by other investigators.
3. MiR-193a as a Therapeutic Agent for Glomerular Disease
miRNAs can be delivered in tissues using viral vectors. miRNAs can be inhibited by using chemically modified miRNAs such as oligonucleotides and locked nucleic acids
[19][77]. Anti-miR-21 is being tested to retard the progression of Alport nephropathy as miR-21 inhibition in experimental models decreased fibrosis and inflammation in glomeruli and interstitium via its effect on PPAR/retinoid X receptor pathway
[20][78]. While several miRNAs are being tested in preclinical studies exploring their impact on podocyte recovery
[21][79] and glomerular diseases
[22][23][80,81], none except anti-miR-21 have been tested in human clinical trials. The mode of delivery of miRNA inhibitor/mimic in human kidneys is unclear; if given systemically, the associated nonspecific side effects of miRNAs are potentially bothersome. On the contrary, oligonucleotide-based therapeutics like small interfering RNAs (siRNAs) targeting alternative complement pathway proteins are being tested in IgA nephropathy patients
[23][81]. Developing and making oligonucleotide-based therapeutics is financially favorable compared to monoclonal antibodies. MiRNA-based therapeutics to treat glomerular diseases will likely see rapid growth in the near future.