Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Mona Zou and Version 1 by Ying Li.
With increasing environmental awareness and consumer demand for high-quality food products, industries are strongly required for technical innovations. The use of various emerging techniques in food processing indeed brings many economic and environmental benefits compared to conventional processes. However, lipid oxidation induced by some “innovative” processes is often “an inconvenient truth”, which is scarcely mentioned in most studies but should not be ignored for the further improvement and optimization of existing processes. Lipid oxidation poses a risk to consumer health, as a result of the possible ingestion of secondary oxidation products.
- advantages
- disadvantages
- green food processing
- lipid degradation
- inducing factors
- innovative techniques
- oxidative mechanism
1. Ultrasound
1.1. Principle
Ultrasound has found numerous applications in the food industry, such as processing, extraction, emulsification, preservation, homogenization, etc. [33][1]. Ultrasound (US) refers to mechanical waves which have the property of spreading in elastic media such as liquids [34][2]. The ultrasonic wave is mainly characterized by four physical parameters, namely the frequency (Hertz), ultrasonic power (W), wavelength (cm) and ultrasonic intensity (W·cm−2). It is worth mentioning that ultrasonic intensity (UI) is directly related to ultrasonic power (UI = P/S; P: power (W) and S: the emitting surface (cm2)).
US frequencies range between 20 kHz and 10 MHz, above the human hearing range (from 16 Hz to 20 kHz). High frequencies (from 2 MHz to 10 MHz) and low ultrasonic power (P < 1 W) are applied in the case of diagnostic US essentially used for therapeutic purposes such as medical imaging. In this power range, there is no destructive effect into the medium. The desired effect is only to characterize the medium by measuring the submitted modification of the ultrasonic wave during its propagation into the medium [32][3]. Power US is characterized by low frequencies (from 20 kHz to 100 kHz) and high ultrasonic power (P > 10 W). Contrarily to diagnostic US, high power promotes physical and chemical effects by creating sufficient interaction between the ultrasonic wave and the elastic medium. This frequency range is widely valorized in several fields such as food processing and extraction of natural products. Physical impacts are essentially observed at low frequencies (from 20 kHz to 100 kHz), while different chemical impacts can be observed in the extended range of power US frequencies (up to 2 MHz), mainly in the formation of radicals [35][4].
US-induced impacts can be attributed to the cavitation phenomenon referring to bubble formation, growth and implosion during its propagation into an elastic medium [34,36][2][5]. The benefit of the cavitation phenomenon is related to the concentration of acoustic energy in small volumes (bubbles) and its conversion in extreme physical conditions of temperature and pressure. While passing through an elastic medium, a spatial and temporal variation in acoustic pressure is induced into the medium, where an oscillatory movement can therefore be observed on the surface.
Undergoing a succession of compression and rarefaction phases, the medium’s constitutive molecules can be displaced from their equilibrium position. During the compression phase (negative acoustic pressure), intermolecular distance is significantly reduced leading to possible collision with the surrounding molecules. During the rarefaction phase (positive acoustic pressure), intermolecular distance increases dramatically [37][6]. Thus, voids are created between the constitutive molecules once their cohesive forces are exceeded by a higher ultrasonic power. These voids, also called bubbles, are formed from vapors or gases initially present in the elastic medium. Vapors and/or gases entering bubbles are partially expelled during the compression phase, resulting in a final increase in bubble size after many cycles of rarefaction/compression phases. In other words, the bubble volume increases with each cycle until it reaches a critical size. At this stage, bubbles collapse during the compression cycle [34,35,37,38][2][4][6][7]. Bubble implosion results in the creation of hot spots with extreme conditions of temperature (up to 5000 K) and pressure (up to 5000 atm), which explains their extremely high physical and chemical reactivity [34,36,37,38][2][5][6][7].
1.2. Effects on Food Lipids
Although ultrasound is able to produce beneficial modifications in food quality parameters (e.g., viscosity and homogenization), the physicochemical effects of ultrasound treatment might also result in quality impairments of food products by the appearance of off-flavors, modifications in physical parameters and degradation of major and minor compounds. Due to these critical temperature and pressure conditions, allied to the formation of radicals during sonocavitation, some alterations in food components have been reported during ultrasonic treatment. Acoustic cavitation can produce radicals in a liquid medium and molecules such as OH and H radicals can accumulate at the surface of the cavitation bubble, which can be responsible for initiating the formation of degradation products that can also trigger radical chain reactions and provoke substantial quality defects in those products [39][8]. The potential restrictions and/or uses of the chemical effects generated by cavitation phenomena are shown in Figure 51.
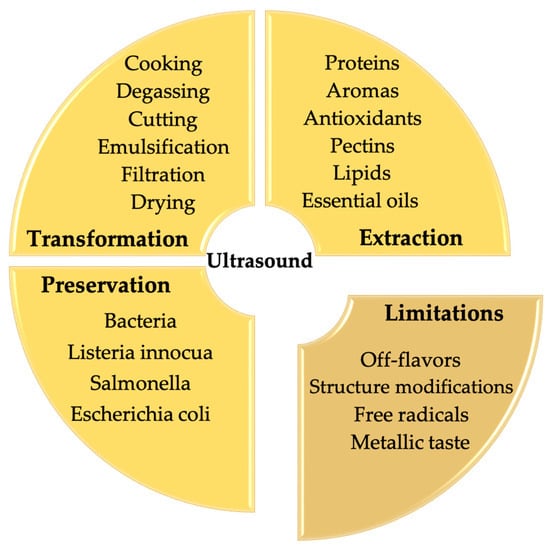
Figure 51. The potential restrictions and/or chemical effects generated by cavitation phenomena.
2. Microwaves
2.1. Principle
Nowadays, microwaves have not only gained in popularity for defrosting, heating or cooking, but are also used in food processing such as drying, thawing, tempering, cooking, baking, sterilization, blanching and extraction. Microwave radiation has many advantages; this process is completed in a few seconds or minutes with high reproducibility, reducing the extraction time and energy normally needed for conventional heating.
Microwaves are electromagnetic waves with a frequency range from 0.3 GHz to 300 GHz, i.e., they span the range of wavelengths from 1 m to 1 cm. Their waves are between radio frequencies and infrared radiation on the electromagnetic spectrum. Industrial applications in food processing have grown steadily since the frequencies of 2.45 GHz and 915 MHz have become more common. Microwaves are composed of an electric and magnetic field and thus represent electromagnetic energy. This energy is a type of innocuous radiation that creates the molecular motion of ions by the rotation of dipoles but has no effect on molecular structure. This dipole rotation comes from alternative movement of polar molecules which try to line up with the electric field. Many collisions due to the agitation of molecules generate energy release, which results in rapid heating. Thus, microwave radiation comes from dissipation of the electromagnetic waves in the irradiated material. The dissipated power in the medium depends on the dielectric properties and the electric field strength.
The mechanisms of microwaves and conventional heating are different. Microwave heating transforms electromagnetic energy into thermal energy, which starts from a heat source and transfers to a medium by conduction, convection or radiation in conventional heating. This phenomenon can be explained by the Fourier heat equation, where ρ, Cp, κ, T and t represent the specific density (kg·m−3), specific heat capacity (J·kg−1·K−1), thermal conductivity (W·m−1·K−1), temperature (K) and time (sec), respectively.
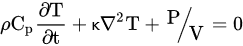
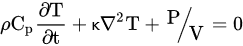
2.2. Effects on Food Lipids
In contrast with conventional heating, microwaves allow a rapid rise in temperature, a volumetric heating, and the maximum temperature of the irradiated material depends only on the rate of heat loss and power applied. The distribution of the electric field is not homogeneous in the irradiated material and “hot spots” appear if heat production is faster than heat transfer. At this moment, for highly viscous media such as oils, the degradation induces oxidative processes to vegetable oils, leading to quality and nutritional losses, as well as lower bioactive properties and physical changes [60][9]. Cerretani et al. [57][10] investigated the effect of microwave radiation on the formation of reactive free radicals that rapidly reacted with atmospheric oxygen to produce secondary oxidation products. The formation of secondary oxidation products in olive oil was determined by testing for the p-anisidine value, which showed a significant increase after 3 min of microwave heating. The peroxide value as another oxidative index was also evaluated, which greatly decreased after up to 6 min of heating. These preliminary results show that microwave energy may induce oxidation in olive oil.
Moreover, Borges et al. [59][11] studied the effects of microwave heating on the composition and physicochemical properties of baru and soybean crude oils. They concluded that both oils became oxidized after 3 min of heating with a 94% decrease in tocopherol content, corresponding to a reduced antioxidant activity by half, and the oxidative stability was reduced by about 72%, accompanied by the loss of its typical yellow coloration. In the same way, Karrar et al. [64][12] investigated the impact of microwave heating on the lipid composition and the oxidative stability of gurum seed oil, whose results showed that triacylglycerol and diacylglycerol decreased with microwave heating (800 W) after 2, 4 and 6 min, respectively, compared with the untreated sample. This same trend was observed for the change in tocopherol content, which has several benefits to overall human health. Another study aimed at evaluating the physicochemical properties and oxidation stability of castor oil using microwave-assisted solvent extraction (MAE) from castor seed [105][13]. The oil from the MAE was more viscous and had a higher acidic value compared to that of the Soxhlet extraction as the reference. The increase in acidic value may be attributed to the hydrolysis of triacylglycerols by microwaves which produce more free fatty acids.
3. Ohmic Heating
3.1. Principle
Ohmic heating is also known as electric resistance heating, which is a technique based on the passage of alternative current (50–100 Hz) through food material in order to generate internal heat (i.e., Joule effect). Parameters such as the voltage and the frequency of electric current and electrical conductivity can affect the characteristics of food components since they determine the heating rate. The first industrial application of ohmic heating began in 1920 with milk pasteurization in a continuous process. This technique is particularly fit for viscous products, liquid foods and the concentration process especially for fruits with a high electrical conductivity value which leads to heating in a few seconds. Many studies revealed that ohmic heating is superior to conventional heating in terms of energy and time saving [106][14]. In addition, the use of low frequencies between 50 and 60 Hz increases electrochemical reactions and the erosion of electrodes, where the contact between the electrodes and the material food is a critical aspect of the process.
The advantages of ohmic heating include uniform and volumetric heating, reduced processing time and thermal damage to thermolabile components like vitamins, bioactive ingredients and color parameters [107][15], as well as non-contact between the food material and hot surfaces. Ohmic heating allows for the conversion of electrical energy into thermal energy, which can be used as an intermittent batch process or in a continuous flow system [108,109][16][17]. Several studies showed that ohmic heating had little effect on the oxidative degradation of vitamin C [110][18], whose degradation depends on the treatment time, the type of electrode and the voltage gradient.
3.2. Effects on Food Lipids
Ohmic heating is unsuitable for foods with low electrical conductivity such as those with a high fat content. Hence, studies concerning the impact of ohmic heating on fatty acid profiles are still scarce. Al-Hilphy et al. [111][19] reported an ohmic-based oil extraction from fish waste, which showed better quality under appropriate processing conditions than the conventional method. Fresh Gac aril is very susceptible to oxidation and degradation; Aamir and Jittanit [112][20] studied the effect of ohmic heating on Gac aril oil extraction in comparison with conventional heating. The experiments were conducted using three extraction stages at 50 °C with the selected ratio of Gac aril powder to the solvent and time for each stage. With the ohmic method, the extraction efficiency and the content of carotenoids in the Gac aril oil were enhanced with the porous and ruptured microstructure of oil-extracted raw material. Kumari et al. [113][21] optimized their process parameters (900 V/m, 85 °C for 10 min) to maximize the recovery of sesame oil. Although the ohmic heating treatment of sesame slightly increased the FFA in the oil, all FFA values were below the maximum permissible limit for all treatment combinations. In addition, Kuriya et al. [114][22] investigated the effect of ohmic heating on the quality of blueberry-flavored dairy desserts, where different electric field strengths (1.82, 3.64, 5.45, 7.30 and 9.1 V/cm) at 60 Hz were used and compared to a conventional heat treatment (90 °C/3 min) as the control. The type of processing and the electrical field had no significant impact on the fatty acid profile.
4. Plasma
4.1. Principle
Plasma is often referred to as the fourth state of matter. It is an ionized gas composed of free electrons, ions, reactive atoms, neutral fractions and photons that are in a metastable state with a net charge of approximately zero. According to the temperature of electrons, plasma can be divided into low-temperature and high-temperature plasma [115][23]. More specifically, low-temperature plasma can be divided into thermal plasma and non-thermal or cold plasma according to its thermodynamic equilibrium [116][24]. Moreover, cold plasma exhibits thermodynamic imbalance at two temperatures, i.e., ions and neutral molecules remain at low temperatures (slightly higher than room temperature), while the temperature of electron gas is about 104 K [117][25]. Therefore, the cold plasma system used in food processing is kept at a relatively low temperature, which is very beneficial to the food processing industry.
Dielectric barrier discharge (DBD) and plasma jets are commonly used in food processing. The DBD device consists of two metal electrodes while at least one electrode is covered by a dielectric barrier, which acts as a stabilizing material to avoid any arc transitions and create a large amount of microdischarge for uniform processing. The plasma jet device consists of two concentric electrodes while the inner electrode is usually connected to power at a high frequency, resulting in the ionization of working gas, which presents as a “jet-like” nozzle [118][26].
4.2. Effects on Food Lipids
Plasma is an emerging food processing technology, among which non-thermal plasma, especially atmospheric plasma, has received widespread attention in the food industry [119][27]. It is an accelerated oxidation technology with great potential to predict lipid oxidation phenomena and/or oxidation stability. This plasma can standardize the control of lipid-accelerated oxidation in complex food matrices with the production of high-concentration active substances such as singlet oxygen, hydroxyl radicals, atomic oxygen, ozone and excited molecular nitrogen [78][28]. Unfortunately, these active substances, and free radicals in particular, can also initiate lipid oxidation by hydrogen abstraction from lipid molecules.
Gas in the electric field can accelerate the movement of charged ions and free electrons. These accelerated particle collisions with other molecules lead to energy sharing, displacement reactions and charge exchange, resulting in several free radicals. When discharging to feed gases containing N2 and O2 molecules, their collision with electrons leads to a series of reactions, forming NxOy, O3 and peroxy dimer. The collision of electrons (e−) with O2 leads to the formation of solitary oxygen atoms in the discharge zone (e− + O2 → 2O + e−), which are then attacked by reaction O2 to produce ozone (O + O2 + M → O3 + M), where M is O, O2 or O3. However, ozone and singlet oxygen will promote potential lipid oxidation in foods. When water is present in the feed gas, it causes OH, H2O2 and H formation, which in turn may inhibit O3 formation [71][29].
Gavahian et al. (2018) [71][29] found that the thiobarbituric acid reactive substance (TBARS) value of brown rice after 20 min of atmospheric plasma treatment was higher than that of white rice, indicating that plasma is more suitable for foods with a relatively low fat content. Bahrami et al. [72][30] showed that treating wheat flour with plasma for 1 or 2 min significantly reduced the content of free fatty acids and phospholipids in the wheat flour, and plasma-treated wheat flour decreased the content of linoleic acid by 100% compared to untreated wheat flour. Thirumdas et al. [120][31] observed that the peroxide content in peanuts and walnuts treated with 60 kV plasma increased by 20% in their production of oxidative rancidity. Lee et al. [73][32] found that dielectric barrier discharge for 10 min did not cause the oxidation effect in packed chicken breast. The oxidation stability of the chicken breast was however better than that of red meat plasma, which might have been related to the higher fat content in the meat. Choi et al. [74][33] found that corona spray discharge caused lipid oxidation, resulting in an increase in the TBARS value during storage while it could improve the sanitary quality of semi-dry squid. The high unsaturated fatty acid content in squid is sensitive to lipid oxidation [121][34], which may be related to primary oxidation products and active substances produced by further plasma reactions. The free fatty acids and other primary oxidation products generated from the drying process make the lipids in squid more susceptible to oxidation by plasma.
5. High Pressure
5.1. Principle
Pressure is a basic thermodynamic variable corresponding to temperature. Thermal effects during a high-pressure process (HPP) can cause changes in material volume and energy [122][35]. Combined net effects during an HPP may be synergistic, antagonistic or superimposed. Reactions such as phase transitions or molecular redirection depend on temperature and pressure, which cannot be treated alone. The previously mentioned HPP principles as follows [123][36].
-
Isostatic principle: Regardless of the geometry and size of the food, the pressure is assumed to be uniform and equal in all directions of the food composition.
-
Le Chatelier’s principle: Any phenomena (phase transition, changes in molecular configuration, chemical reactions) accompanied by a decrease in volume are enhanced by pressure, which will facilitate a system’s transition to the lowest volume.
-
Microscopic ordering principle: An increase in pressure at a constant temperature enhances the order of a given material molecule. Therefore, pressure and temperature antagonize molecular structures and chemical reactions.
-
Arrhenius relationship: As with heat treatment, various reaction rates in the HPP process are also affected by the thermal effect during pressure treatment. Net pressure–heat effects can be synergistic, superimposed or antagonistic.
5.2. Effects on Food Lipids
The most pressure-sensitive biological components are lipid systems [124][37]. Indeed, the melting temperature of triglycerides can increase by more than 10 °C per 100 MPa, and thus lipids in a liquid state at room temperature crystallize under high-pressure treatment [12][38]. Bolumar et al. [22][39] found that free radical formation would not occur at pressures below 400 MPa, which can be considered as a threshold in HPP treatment. The kinetics of free radical formation followed a zero-order reaction at pressures below 600 MPa, whereas that at higher treatment pressures was more aligned with a first-order reaction with a reaction rate of 0.016–0.07 μM/min [125][40].
Pressure affects not only the physical properties of food components (e.g., surface tension, density, viscosity and thermal properties, etc.) and dynamic equilibrium processes, but also the rate of lipid oxidation by slowing down or accelerating the reaction. Hebishy et al. [84][41] observed a higher oxidation rate for emulsions treated by an ultrahigh pressure of 200 MPa compared to those treated by 100 MPa, especially for those containing 1% or 2% of whey protein isolate, which may have been due to the decreased ability of whey protein to protect oil droplets. With the increasing pressure in the ultrahigh pressure treatment, the temperature at the outlet of the homogeneous valve increased, resulting in the over-processing phenomenon. Whey proteins were partially denatured or aggregated, leading to large polymeric dissociation, which could allow more proteins to gather on the droplet surface and prevent oxidation better [85][42]. Pereda et al. [87][43] found that the content of malondialdehyde and hexanal was much lower in milk under 300 MPa compared to that of 200 MPa. Wang et al. [86][44] also found that the TBARS values of treated fat samples at 400 MPa and 600 MPa were much higher than those at 200 MPa, indicating that lipid oxidation increased with pressure.
Although the temperature generated by high-pressure processing is considered low, it is sufficient enough to affect various nutrients and bioactive molecules [2][45]. The emulsification of multiple oils (i.e., sunflower, camel and fish oils) by microfluidization at the pressure of 21–138 MPa using sodium caseate as an emulsifier could lead to an increase in oxidation stability [87][43]. The increased temperature of water-in-oil emulsion during pressure treatment could lead to the binding of lipids to proteins during storage, resulting in a reduction of oxidation products. Bolumar et al. [22][39] found thresholds for the formation of free radicals at 25 °C and 400 MPa and 5 °C and 500 MPa, respectively. Above these thresholds, free radical formation increased with the increasing pressure, temperature and time. It is believed that the synergistic effects of high pressure and temperature could promote lipid oxidation.
In addition, there are many factors affecting oxidation, such as the oil content, physical structure of emulsion (e.g., size and specific surface area of droplets), emulsifier and emulsion type, etc. [83,126][46][47]. Fuentes et al. [88][48] reported the oxidative stability difference in two dry-cured ham types under a high-pressure treatment of 600 MPa, namely in the flank (lower fat content) and hip (higher fat content), indicating that unsaturated lipids in the flanking samples were more easily oxidized corresponding to their high TBARS value. Atares et al. [80][49] used a high-pressure jet homogenizer of 30 MPa to determine the structure and oxidative stability of water-in-oil emulsions prepared with sunflower oil in the presence of the flavonoids rutin and whey protein as emulsifiers. The droplet size decreased after high-pressure homogenization, whereas the emulsion structure stability increased, thus reducing lipid oxidation. Nakaya et al. [127][50] found that the oxidation stability of lipids in an emulsion could be enhanced by reducing the droplet size. Phoon et al. [81][51] used high-pressure homogenization (0.1~137.9 MPa) to form a water-in-oil emulsion (4%, w/v), which showed a poor oxidation stability due to its larger droplet size when exposed to oxygen directly. Ultrahigh-pressure homogenization is a novel antioxidant technique for the production of fine, stable submicron emulsions [128][52]. Soybean oil and conjugated linoleic acid emulsions (20%, v/v) containing soy protein isolate (4%, w/v) as an emulsifier were studied [82][53], indicating that the emulsion treated by ultrahigh-pressure homogenization (100~300 MPa) had the smallest particle size with the best oxidation stability. Furthermore, lipid oxidation decreased with increasing oil content under constant pressure according to the change in the TBARS value, which is consistent with previous findings [129][54]. This may have been due to the fact that the water-soluble pro-oxidant components decreased proportionally with the increased oil phase in the emulsion, thus reducing the number of free radicals and slowing down lipid oxidation [130][55]. Compared to other oil content, emulsions with 10% of oil content treated at an ultrahigh pressure also had poor physical stability, which might have been due to their link with oxidation stability [131][56]. The mechanism of HPP-induced cholesterol oxidation remains unclear. The most supported hypothesis is related to cell membrane damage, which can induce free radical formation through the synergistic action of denatured proteins [132][57]. Furthermore, applying very high pressure (>800 MPa) can also form free radicals and promote lipid peroxidation, resulting in cholesterol oxidation [125][40]. It is believed that the increase in the oxidation rate may be due to the increase in the interface area, which leads to the increase in contact between the oil and peroxide.
6. Pulse Electric Field
6.1. Principle
PEF technology applies a high voltage pulse in a specific and short amount of time, resulting in a high electric field with electroporation phenomena occurring in the treated material placed between two electrodes [133][58]. A transmembrane potential difference is formed on the cell membrane under the action of an applied electric field. When the electric field strength of the transmembrane exceeds the threshold, the voltage shrinkage force causes a local dielectric breakdown of the membrane, resulting in a pore as a conductive channel [134][59]. Due to high electric field pulses, the cell membrane increases membrane permeability by expanding existing pores or generating new ones, which may be permanent or temporary depending on the operating conditions [29][60]. The mechanism of electroporation is mainly based on the voltage contraction force that affects the cell membrane. Hence, the pulsed electric field technique is considered as a pretreatment process for the disintegration of vegetative organisms [135][61], which illustrates the electrical, reversible and irreversible breakdown of the cell membrane.
6.2. Effects on Food Lipids
PEF is a non-thermal food preservation technology mainly used in liquids. Compared to traditional hot barrel sterilization, PEF can inactivate most pathogenic or spoilage microorganisms, which has the advantages of maintaining food freshness effectively, having an impact on enzymatic activity and is energy-saving. Minimizing the loss of taste, color, texture, nutrition and thermal-sensitive functional components in food has attracted increasing attention in recent years [9,136,137,138][62][63][64][65]. PEF is among the emerging technologies that have been successfully applied in various low-viscosity liquid foods such as milk, soy milk, pea soup, egg liquid and juice beverages [89][66]. However, few studies concerning the effects of PEF on food composition have been reported, especially in food lipids [139][67]. Therefore, understanding the role of PEF technology in electrochemical reactions and lipid oxidation is necessary for further development of the food processing industry [95][68].
PEF treatment can change the permeability of cells, which makes meat components such as lipids easier to oxidize or to promote the reaction between enzymes and their substrates. It can also change fatty acids and volatile components and ultimately affects the shelf life of food [93][69]. Moreover, Pataro et al. [140][70] showed that metal ions released from pulsed electric fields led to electrode contamination or corrosion, and even lipid oxidation at the end. Zeng et al. [89][66] observed that the acidic value of PEF-treated peanut oil after storage at 40 °C for 100 days was lower than that of untreated peanut oil, while the carbonyl value during this storage period decreased with the increase in the electric field intensity, indicating that PEF treatment could inhibit the rate of lipid oxidation. Arroyo et al. [90][71] found that the malondialdehyde content of PEF-treated fresh frozen chicken breast increased but there was no significant difference in the TBARS value for different conditions. Cortes et al. [91][72] also noted that the peroxidase of PEF-treated samples was partially inactivated while the TBARS value was not significantly changed.
Furthermore, Ma et al. [92][73] found that PEF-treated lamb meat would not produce lipid oxidation immediately. However, the malondialdehyde content in the treated sample after 7 days of storage was higher than that in the control, though the product quality was still acceptable (<2 mg malondialdehyde/kg sample). Notwithstanding, Faridnia et al. [93][69] found that the lipid oxidation of PEF-treated beef muscles was significantly enhanced, where the TBARS value was higher than that of the non-PEF treated samples. PEF treatment made thawed-from-frozen meat more prone to lipid autoxidation caused by the release of metal ions in iron complexes. The thawed-from-frozen samples accumulated the most malondialdehyde content after a storage period of 18 days. High-voltage PEF-treated boned beef samples exhibited a higher lipid oxidation rate compared to those treated with low-voltage PEF at the end of the storage period [3][74], which is probably because of the high thermal energy generated during the high-voltage PEF treatment that could reduce the antioxidant capacity of meat and accelerate the lipid oxidation rate during storage. Moreover, no significant effect was found on the acidic value of PEF-treated oleic acid and lecithin samples after storage [95,96][68][75]. Nevertheless, the change in both the peroxide value and carbonyl value at different degrees was influenced by the electric field intensity and storage time, indicating that PEF treatment did induce the oxidation of oleic acid and lecithin.
7. Radiation
7.1. Principle
The effects of radiation can be divided into direct and indirect effects. The direct effect is due to the nonspecific collision of radiation photons with atoms in microbial molecules. Radiation disintegrates key biomolecules such as DNA, RNA, enzymes and membrane proteins [141][76]. It also induces the formation of DNA photoproducts, namely cyclobutane pyrimidine dimer and pyrimidine (6–4) pyrimidone photoproducts, which inhibit transcription and replication and inactivate microorganisms [142,143][77][78]. The indirect effect is due to the effect of free radicals produced during irradiation hydrolysis. Ionizing radiation can generate sufficiently high energy to activate chemical reactions in many food systems. Radiation first ionizes one electron in the effluent, producing highly active substances such as hydroxyl radicals and hydrogen peroxide, and then forms many intermediates which can react with each other or with other components in the system. Many intermediates produced during this time have high chemical activity [144][79]. Therefore, the indirect effect of irradiation on microbial inactivation depends on the water availability in food [141][76].
7.2. Effects on Food Lipids
Electromagnetic waves (e.g., visible, x, γ, ultraviolet, infrared, etc.) and electrons can be used in food processing with the advantages of having uniform heating, high heat transfer efficiency, less mass loss, being energy-saving, and having a prolonged shelf life and improved safety. It has been reported that the shorter the wavelength, the better the thermal penetration effect [98][80]. The free radicals formed by irradiation have an important effect on the oxidative stability of foods with high fat content, but generally, they have no effect on the nutritional value of foods. Similar to the results of lipid changes observed using conventional methods, irradiation accelerates oxidative decay in foods [145][81]. Food products with either a higher lipid or unsaturated fatty acid content are more prone to oxidation reaction, which is mainly caused by free radicals formed in the indirect action of radiation [146][82]. The higher the irradiation dose, the higher the excitation level, and thus more free radicals are produced to easily enhance lipid oxidation and color change [147][83].
The lipid oxidation rate increases with the radiation dose. A significant positive correlation between the radiation dose and peroxide value was found with a correlation coefficient of 0.908 [97][84]. The peroxide value of peanut oil extracted from infrared radiation-treated seeds was significantly higher than that from original seeds [98][80], which may be due to the temperature increase during the roasting process. According to Lee et al.’s study [148][85], the radiation dose of 5.0 kGy greatly increased the oxidation of soybean oil, cottonseed oil, corn oil and linoleic acid. The concentration of both primary and secondary oxidation products increased with the increase in γ radiation dose [99][86]. Both primary and secondary oxidation products accumulated in peanut oil under a γ radiation of 8 kGy, where the content of secondary oxidation products increased faster [100][87]. Cashew nuts (Anacardium occidentale L.) radiated at higher doses (7 kGy) could be oxidized to form aldehydes and ketones as well [102][88]. The content of these volatile secondary oxidation products was also found to increase significantly in peanut and pistachio oils using the same radiation dose [103][89].
The oxidation stability index is affected by many factors, such as fatty acid composition and antioxidant content. Total antioxidant capacity increases as the roasting temperature increases. Hence, the storage stability of peanut oil from an infrared radiation pre-baking treatment significantly improved compared to the control [98][80]. Similarly, some Maillard reaction products generated from heating treatment can also improve the antioxidant capacity of oil [149][90]. However, γ radiation shortens the induction period of crude peanut oil and reduces the oxidative stability, though the total tocopherol content is positively correlated with the induction period [99][86].
Radiation can also cause a content change in endogenous antioxidants in oils to some extent, like tocopherols and phenolic compounds. The polyphenolic content of peanut oil extracted from infrared radiation roasted seeds increased by 62.20% whereas the contents of total tocopherol and three tocopherol congeners decreased significantly compared to oils from raw peanuts [98][80]. The degradation of tocopherol exceeded the oxidative protection of Maillard reaction products when the temperature increased from 147 °C to 157 °C. The decrease in γ-tocopherol content was affected differently by the instantaneous γ radiation of 5.0 kGy [99][86]. The loss of α-tocopherol in soybean oil was as high as 92.3% with γ radiation of 3.0 KGy [103][89]. Irradiation could significantly decrease the tocopherol content, among which α- and δ-tocopherol degraded the most while γ-tocopherol resistance to degradation was the best [104][91].
References
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835.
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196.
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593–606.
- Mason, T.J.; Paniwnyk, L.; Chemat, F. Ultrasound as a preservation technology. In Food Preservation Techniques; Zeuthen, P., Bøgh-Sørensen, L., Eds.; Woodhead Publishing: Sawston, UK, 2003; pp. 303–337.
- Bermúdez-aguirre, D.; Mobbs, T.; Barbosa-cánovas, G.V. Ultrasound technologies for food and bioprocessing. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G., Weiss, J., Eds.; Springer Science: Berlin/Heidelberg, Germany, 2011; pp. 65–105.
- Vyas, S.; Ting, Y.P. A review of the application of ultrasound in bioleaching and insights from sonication in (bio)chemical processes. Resources 2018, 7, 3.
- Wu, T.Y.; Guo, N.; Teh, C.Y.; Hay, J.X.W. Theory and fundamentals of ultrasound interfacial region. Chem. Eng. J. 2013, 2, 5–9.
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr. Polym. 2005, 60, 175–184.
- Malheiro, R.; Casal, S.; Lamas, H.; Bento, A.; Pereira, J.A. Can tea extracts protect extra virgin olive oil from oxidation during microwave heating? Food Res. Int. 2012, 48, 148–154.
- Cerretani, L.; Bendini, A.; Rodriguez-Estrada, M.T.; Vittadini, E.; Chiavaro, E. Microwave heating of different commercial categories of olive oil: Part I. effect on chemical oxidative stability indices and phenolic compounds. Food Chem. 2009, 115, 1381–1388.
- Borges, T.H.; Malheiro, R.; de Souza, A.M.; Casal, S.; Pereira, J.A. Microwave heating induces changes in the physicochemical properties of baru (Dipteryx alata Vog.) and soybean crude oils. Eur. J. Lipid Sci. Technol. 2015, 117, 503–513.
- Karrar, E.; Sheth, S.; Wei, W.; Wang, X. Effect of microwave heating on lipid composition, oxidative stability, color value, chemical properties, and antioxidant activity of gurum (Citrullus lanatus var. Colocynthoide) seed oil. Biocatal. Agric. Biotechnol. 2020, 23, 101504.
- Ibrahim, N.A.; Zaini, M.A.A. Microwave-assisted solvent extraction of castor oil from castor seeds. Chin. J. Chem. Eng. 2018, 26, 2516–2522.
- Gavahian, M.; Tiwari, B.K.; Chu, Y.H.; Ting, Y.; Farahnaky, A. Food texture as affected by ohmic heating: Mechanisms involved, recent findings, benefits, and limitations. Trends Food Sci. Technol. 2019, 86, 328–339.
- Cappato, L.P.; Ferreira, M.V.S.; Guimaraes, J.T.; Portela, J.B.; Costa, A.L.R.; Freitas, M.Q.; Cunha, R.L.; Oliveira, C.A.F.; Mercali, G.D.; Marzack, L.D.F.; et al. Ohmic heating in dairy processing: Relevant aspects for safety and quality. Trends Food Sci. Technol. 2017, 62, 104–112.
- Jaeger, H.; Roth, A.; Toepfl, S.; Holzhauser, T.; Engel, K.H.; Knorr, D.; Vogel, R.F.; Bandick, N.; Kulling, S.; Heinz, V.; et al. Opinion on the use of ohmic heating for the treatment of foods. Trends Food Sci. Technol. 2016, 55, 84–97.
- Vikram, V.B.; Ramesh, M.N.; Prapulla, S.G. Thermal degradation kinetics of nutrients in orange juice heated by electromagnetic and conventional methods. J. Food Eng. 2005, 69, 31–40.
- Louarme, L.; Billaud, C. Evaluation of ascorbic acid and sugar degradation products during fruit dessert processing under conventional or ohmic heating treatment. LWT Food Sci. Technol. 2012, 49, 184–187.
- Al-Hilphy, A.R.; Al-Mtury, A.A.; Al-Shatty, S.M.; Hussain, Q.N.; Gavahian, M. Ohmic heating as a by-product valorization platform to extract oil from Carp (Cyprinus carpio) viscera. Food Bioprocess. Technol. 2022, 15, 2515–2530.
- Aamir, M.; Jittanit, W. Ohmic heating treatment for Gac aril oil extraction: Effects on extraction efficiency, physical properties and some bioactive compounds. Innov. Food Sci. Emerg. Technol. 2017, 41, 224–234.
- Kumari, K.; Mudgal, V.D.; Viswasrao, G.; Srivastava, H. Studies on the effect of ohmic heating on oil recovery and quality of sesame seeds. J. Food Sci. Technol. 2016, 53, 2009–2016.
- Kuriya, S.P.; Silva, R.; Rocha, R.S.; Guimarães, J.T.; Balthazar, C.F.; Pires, R.P.S.; Tavares Filho, E.R.; Pimentel, T.C.; Freitas, M.Q.; Cappato, L.P.; et al. Impact assessment of different electric fields on the quality parameters of blueberry flavored dairy desserts processed by ohmic heating. Food Res. Int. 2020, 134, 109235.
- Misra, N.N.; Tiwari, B.K.; Raghavarao KS, M.S.; Cullen, P.J. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170.
- Pan, Y.; Cheng, J.H.; Sun, D.W. Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1312–1326.
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91.
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4.
- Lee, J.; Jo, K.; Lim, Y.; Jeon, H.J.; Choe, J.H.; Jo, C.; Jung, S. The use of atmospheric pressure plasma as a curing process for canned ground ham. Food Chem. 2018, 240, 430–436.
- Van Durme, J.; Vandamme, J. Non-thermal plasma as preparative technique to evaluate olive oil adulteration. Food Chem. 2016, 208, 185–191.
- Gavahian, M.; Chu, Y.H.; Mousavi Khaneghah, A.; Barba, F.J.; Misra, N.N. A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci. Technol. 2018, 77, 32–41.
- Bahrami, N.; Bayliss, D.; Chope, G.; Penson, S.; Perehinec, T.; Fisk, I.D. Cold plasma: A new technology to modify wheat flour functionality. Food Chem. 2016, 202, 247–253.
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold plasma: A novel non-thermal technology for food processing. Food Biophys. 2014, 10, 1–11.
- Lee, H.; Yong, H.I.; Kim, H.J.; Choe, W.; Yoo, S.J.; Jang, E.J.; Jo, C. Evaluation of the microbiological safety, quality changes, and genotoxicity of chicken breast treated with flexible thin-layer dielectric barrier discharge plasma. Food Sci. Biotechnol. 2016, 25, 1189–1195.
- Choi, S.; Puligundla, P.; Mok, C. Impact of corona discharge plasma treatment on microbial load and physicochemical and sensory characteristics of semi-dried squid (Todarodes pacificus). Food Sci. Biotechnol. 2017, 26, 1137–1144.
- Van Durme, J.; Nikiforov, A.; Vandamme, J.; Leys, C.; De Winne, A. Accelerated lipid oxidation using non-thermal plasma technology: Evaluation of volatile compounds. Food Res. Int. 2014, 62, 868–876.
- Gupta, R.; Mikhaylenko, G.; Balasubramaniam, V.M.; Tang, J. Combined pressure-temperature effects on the chemical marker (4-hydroxy-5-methyl-3(2H)-furanone) formation in whey protein gels. LWT Food Sci. Technol. 2011, 44, 2141–2146.
- Balasubramaniam, V.M.B.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure-based technologies in the food industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462.
- Rivalain, N.; Roquain, J.; Demazeau, G. Development of high hydrostatic pressure in biosciences: Pressure effect on biological structures and potential applications in Biotechnologies. Biotechnol. Adv. 2010, 28, 659–672.
- Schaich, K.M. Thinking outside the classical chain reaction box of lipid oxidation. Lipid Technol. 2012, 24, 55–58.
- Bolumar, T.; Skibsted, L.H.; Orlien, V. Kinetics of the formation of radicals in meat during high pressure processing. Food Chem. 2012, 134, 2114–2120.
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G.V. Effects of high pressure processing on lipid oxidation: A review. Innov. Food Sci. Emerg. Technol. 2014, 22, 1–10.
- Hebishy, E.; Zamora, A.; Buffa, M.; Blasco-Moreno, A.; Trujillo, A.J. Characterization of whey protein oil-in-water emulsions with different oil concentrations stabilized by ultra-high pressure homogenization. Processes 2017, 5, 6.
- Hebishy, E.; Buffa, M.; Guamis, B.; Blasco-Moreno, A.; Trujillo, A.J. Physical and oxidative stability of whey protein oil-in-water emulsions produced by conventional and ultra high-pressure homogenization: Effects of pressure and protein concentration on emulsion characteristics. Innov. Food Sci. Emerg. Technol. 2015, 32, 79–90.
- Pereda, J.; Ferragut, V.; Quevedo, J.M.; Guamis, B.; Trujillo, A.J. Effects of ultra-high-pressure homogenization treatment on the lipolysis and lipid oxidation of milk during refrigerated storage. J. Agric. Food Chem. 2008, 56, 7125–7130.
- Wang, Q.; Zhao, X.; Ren, Y.; Fan, E.; Chang, H.; Wu, H. Effects of high pressure treatment and temperature on lipid oxidation and fatty acid composition of yak (Poephagus grunniens) body fat. Meat Sci. 2013, 94, 489–494.
- Pérez-Andrés, J.M.; Charoux, C.M.G.; Cullen, P.J.; Tiwari, B.K. Chemical modifications of lipids and proteins by nonthermal food processing technologies. J. Agric. Food Chem. 2018, 66, 5041–5054.
- Fernandez-Avila, C.; Trujillo, A.J. Ultra-high pressure homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem. 2016, 209, 104–113.
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977.
- Fuentes, V.; Utrera, M.; Estévez, M.; Ventanas, J.; Ventanas, S. Impact of high pressure treatment and intramuscular fat content on colour changes and protein and lipid oxidation in sliced and vacuum-packaged Iberian dry-cured ham. Meat Sci. 2014, 97, 468–474.
- Atarés, L.; Marshall, L.J.; Akhtar, M.; Murray, B.S. Structure and oxidative stability of oil in water emulsions as affected by rutin and homogenization procedure. Food Chem. 2012, 134, 1418–1424.
- Nakaya, K.; Ushio, H.; Matsukawa, S.; Shimizu, M.; Ohshima, T. Effects of droplet size on the oxidative stability of oil-in-water emulsions. Lipids 2005, 40, 501–507.
- Phoon, P.Y.; Paul, L.N.; Burgner, J.W.; San Martin-Gonzalez, M.F.; Narsimhan, G. Effect of cross-linking of interfacial sodium caseinate by natural processing on the oxidative stability of oil-in-water (O/W) emulsions. J. Agric. Food Chem. 2014, 62, 2822–2829.
- Martinez-Monteagudo, S.I.; Saldaña, M.D.A. Chemical reactions in food systems at high hydrostatic pressure. Food Eng. Rev. 2014, 6, 105–127.
- Fernandez-Avila, C.; Trujillo, A.J. Enhanced stability of emulsions treated by ultra-High pressure homogenization for delivering conjugated linoleic acid in Caco-2 cells. Food Hydrocoll. 2017, 71, 271–281.
- Sun, C.; Gunasekaran, S. Effects of protein concentration and oil-phase volume fraction on the stability and rheology of menhaden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll. 2009, 23, 165–174.
- Kargar, M.; Spyropoulos, F.; Norton, I.T. The effect of interfacial microstructure on the lipid oxidation stability of oil-in-water emulsions. J. Colloid. Interface Sci. 2011, 357, 527–533.
- Fernández-Ávila, C.; Escriu, R.; Trujillo, A.J. Ultra-High Pressure Homogenization enhances physicochemical properties of soy protein isolate-stabilized emulsions. Food Res. Int. 2015, 75, 357–366.
- Bernsdorff, C.; Wolf, A.; Winter, R.; Gratton, E. Effect of hydrostatic pressure on water penetration and rotational dynamics in phospholipid-cholesterol bilayers. Biophys. J. 1997, 72, 1264–1277.
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata PE, S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green. Chem. 2020, 22, 2325–2353.
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184.
- Gómez, B.; Munekata PE, S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol PC, B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105.
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging technologies in food processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235.
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282.
- Donsì, F.; Ferrari, G.; Fruilo, M.; Pataro, G. Pulsed electric field-assisted vinification of aglianico and piedirosso grapes. J. Agric. Food Chem. 2010, 58, 11606–11615.
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends Food Sci. Technol. 2009, 20, 544–556.
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423.
- Zeng, X.A.; Han, Z.; Zi, Z.H. Effects of pulsed electric field treatments on quality of peanut oil. Food Control 2010, 21, 611–614.
- Vallverdú-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventos, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Effects of pulsed electric fields on the bioactive compound content and antioxidant capacity of tomato fruit. J. Agric. Food Chem. 2012, 60, 3126–3134.
- Zhao, W.; Yang, R.; Liang, Q.; Zhang, W.; Hua, X.; Tang, Y. Electrochemical reaction and oxidation of lecithin under pulsed electric fields (PEF) processing. J. Agric. Food Chem. 2012, 60, 12204–12209.
- Faridnia, F.; Ma, Q.L.; Bremer, P.J.; Burritt, D.J.; Hamid, N.; Oey, I. Effect of freezing as pre-treatment prior to pulsed electric field processing on quality traits of beef muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40.
- Pataro, G.; Falcone, M.; Donsì, G.; Ferrari, G. Metal release from stainless steel electrodes of a PEF treatment chamber: Effects of electrical parameters and food composition. Innov. Food Sci. Emerg. Technol. 2014, 21, 58–65.
- Arroyo, C.; Eslami, S.; Brunton, N.P.; Arimi, J.M.; Noci, F.; Lyng, J.G. An assessment of the impact of pulsed electric fields processing factors on oxidation, color, texture, and sensory attributes of turkey breast meat. Poult. Sci. 2015, 94, 1088–1095.
- Cortés, C.; Esteve, M.J.; Frígola, A.; Torregrosa, F. Quality characteristics of horchata (a Spanish vegetable beverage) treated with pulsed electric fields during shelf-life. Food Chem. 2005, 91, 319–325.
- Ma, Q.; Hamid, N.; Oey, I.; Kantono, K.; Faridnia, F.; Yoo, M.; Farouk, M. Effect of chilled and freezing pre-treatments prior to pulsed electric field processing on volatile profile and sensory attributes of cooked lamb meats. Innov. Food Sci. Emerg. Technol. 2016, 37, 359–374.
- Khan, A.A.; Randhawa, M.A.; Carne, A.; Mohamed Ahmed, I.A.; Barr, D.; Reid, M.; Bekhit AE, D.A. Effect of low and high pulsed electric field on the quality and nutritional minerals in cold boned beef M. longissimus et lumborum. Innov. Food Sci. Emerg. Technol. 2017, 41, 135–143.
- Wang, Y.; Wang, Q.; Artz, W.E.; Padua, G.W. Fourier transform infrared spectra of drying oils treated by irradiation. J. Agric. Food Chem. 2008, 56, 3043–3048.
- Tahergorabi, R.; Matak, K.E.; Jaczynski, J. Application of electron beam to inactivate Salmonella in food: Recent developments. Food Res. Int. 2012, 45, 685–694.
- Hinds, L.M.; O’Donnell, C.P.; Akhter, M.; Tiwari, B.K. Principles and mechanisms of ultraviolet light emitting diode technology for food industry applications. Innov. Food Sci. Emerg. Technol. 2019, 56, 102153.
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and applications of non-thermal technologies and alternative chemical compounds in meat and fish. Crit. Rev. Food Sci. Nutr. 2020, 61, 1163–1183.
- Zanardi, E.; Caligiani, A.; Novelli, E. New insights to detect irradiated food: An overview. Food Anal. Methods 2018, 11, 224–235.
- Deng, B.X.; Li, B.; Li, X.D.; Zaaboul, F.; Jiang, J.; Li, J.W.; Li, Q.; Cao, P.R.; Liu, Y.F. Using short-wave infrared radiation to improve aqueous enzymatic extraction of peanut oil: Evaluation of peanut cotyledon microstructure and oil quality. Eur. J. Lipid Sci. Technol. 2018, 120, 1700285.
- Stevenson, M.H. Nutritional and other implications of irradiating meat. Proc. Nutr. Soc. 1994, 53, 317–325.
- Rodrigues, B.L.; da Costa, M.P.; da Silva Frasão, B.; da Silva, F.A.; Mársico, E.T.; da Silveira Alvares, T.; Conte-Junior, C.A. Instrumental texture parameters as freshness indicators in five farmed Brazilian freshwater fish species. Food Anal. Methods 2017, 10, 3589–3599.
- Canto, A.C.V.C.S.; Costa-Lima, B.R.C.; Suman, S.P.; Monteiro, M.L.G.; Viana, F.M.; Salim, A.P.A.A.; Nair, M.N.; Silva, T.J.P.; Conte-Junior, C.A. Color attributes and oxidative stability of longissimus lumborum and psoas major muscles from Nellore bulls. Meat Sci. 2016, 121, 19–26.
- Braunrath, R.; Isnardy, B.; Solar, S.; Elmadfa, I. Impact of α-, γ-, and δ-tocopherol on the radiation induced oxidation of rapeseed oil triacylglycerols. Radiat. Phys. Chem. 2010, 79, 764–769.
- Lee, K.H.; Yook, H.S.; Lee, J.W.; Park, W.J.; Kim, K.S.; Byun, M.W. Quenching mechanism and kinetics of ascorbyl palmitate for the reduction of the gamma irradiation-induced oxidation of oils. J. Am. Oil Chem. Soc. 1999, 76, 921–925.
- De Camargo, A.C.; de Souza Vieira, T.M.F.; Regitano-D’Arce, M.A.B.; de Alencar, S.M.; Calori-Domingues, M.A.; Canniatti-Brazaca, S.G. Gamma radiation induced oxidation and tocopherols decrease in in-shell, peeled and blanched peanuts. Int. J. Mol. Sci. 2012, 13, 2827–2845.
- Bhatti, I.A.; Ashraf, S.; Shahid, M.; Asi, M.R.; Mehboob, S. Quality index of oils extracted from γ-irradiated peanuts (Arachis hypogaea L.) of the golden and bari varieties. Appl. Radiat. Isot. 2010, 68, 2197–2201.
- Mexis, S.F.; Kontominas, M.G. Effect of γ-irradiation on the physicochemical and sensory properties of cashew nuts (Anacardium occidentale L.). LWT Food Sci. Technol. 2009, 42, 1501–1507.
- Lalas, S.; Gortzi, O.; Tsaknis, J.; Sflomos, K. Irradiation effect on oxidative condition and tocopherol content of vegetable oils. Int. J. Mol. Sci. 2007, 8, 533–540.
- Vhangani, L.N.; Wyk, J.V. Antioxidant activity of Maillard reaction products (MRPs) in a lipid-rich model system. Food Chem. 2016, 208, 301–308.
- Lakritz, L.; Fox, J.B.; Hampson, J.; Richardson, R.; Kohout, K.; Thayer, D.W. Effect of gamma radiation on levels of α-tocopherol in red meats and turkey. Meat Sci. 1995, 41, 261–271.
More
