You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Rostislav E Trifonov and Version 2 by Rita Xu.
Tetrazole heterocycle is a promising scaffold in drug design, and it is incorporated into active pharmaceutical ingredients of medications of various actions: hypotensives, diuretics, antihistamines, antibiotics, analgesics, and others. This heterocyclic system is metabolically stable and easily participates in various intermolecular interactions with different biological targets through hydrogen bonding, conjugation, or van der Waals forces.
- tetrazoles
- antidiabetic agents
- type 2 diabetes mellitus
- peroxisome proliferator-activated receptors (PPARs) agonists
1. Introduction
Diabetes mellitus is an incurable disease defined by a metabolic disorder with hyperglycemia, contributing to a number of dangerous diseases: hypertension, thrombosis, neurodegenerative disorders, and so on. Hyperglycemia may be due to poor cellular susceptibility to insulin (insulin resistance) or type 2 diabetes mellitus (T2DM), as well as insufficient secretion of insulin by the pancreas (type 1 diabetes mellitus). Currently, the number of T2DM cases is constantly increasing, and the disease is now considered to be one of the non-transmittable chronic disease epidemics. The problem affects different populations, genders, and ages. Overall, T2DM accounts for about 90% of all cases of diabetes mellitus. To date, there are hundreds of millions of known documented cases of T2DM in the world. Note that not all cases of diabetes are reliably documented. According to International Diabetes Federation (IDF) data, the total number of people living with diabetes is projected to rise to 643 million by 2030 and 783 million by 2045 [1][2][1,2]. Type 1 diabetes mellitus can be controlled with the external administration of insulin. In contrast, a direct injection of insulin in the case of T2DM will not significantly reduce blood glucose. Here, drug therapy is required to target the various biological mechanisms responsible for metabolic processes. A great deal of studies have been dedicated to the development of such drugs, and these studies are being intensively developed [3].
A large number of natural compounds with antihyperglycemic effects are known [4][5][4,5], but it is the synthetic drugs that are most widely used in the treatment of diabetes. Dozens of the biological targets of low-molecular antidiabetic agents are well recognized: the incretin hormones Glucagon-like Peptide-1 (GLP-1) and Glucose-Dependent Insulinotropic Polypeptide (GIP) themselves; their regulators such as Dipeptidyl Peptidase-4 (DPP-4); G protein-Coupled Receptors (GPCRs) such as GPR40, GPR120, and GPR119; Glucose Metabolism Pathway-Based Targets, such as Glucose Kinase (GK), Protein Kinase B (AKT/PKB); Insulin-Based Targets like Protein Tyrosine Phosphatase 1B (PTP1B); and other types of targets like Sodium-Glucose Cotransporter Protein-2 (SGLT-2), Peroxisome Proliferator-Activated Receptors (PPARs), etc. [6]. Antidiabetic drugs can have fundamentally different chemical structures, and they can hardly be assigned to a certain type. As can be seen, for example, from Figure 1, the following chemical compounds of different classes can be used as hypoglycemic agents: biguanides, sulfonylureas, thiazolidinediones, pyrimidines, purines, sugars, oligonucleotides, peptides, and many others [7][8][7,8]. In addition, other multiple medications are commonly used in combination with antidiabetic drugs to suppress secondary effects associated with T2DM and high blood sugar levels: cardiovascular and neurological agents, immunomodulators, and many others. Thus, medical treatments for T2DM are complex processes, and drug therapy, in combination with proper diet and lifestyle, can significantly improve the patient’s condition and reduce the dangerous consequences of hyperglycemia. Nevertheless, to date, there are no universal and highly effective drugs, either of natural or synthetic origin, to cure T2DM. The known drugs have a number of side effects, which has even led to a ban on the use of some of them [7]. Therefore, the development of more effective medications for the treatment of T2DM remains very important.
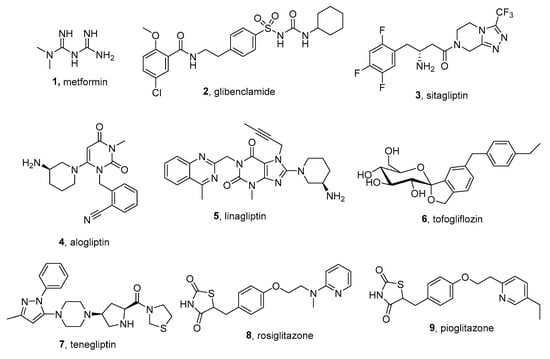
Figure 1. Some low-molecular oral antidiabetic medications: 1—metformin (known since 1922, decreases glucose production in the liver, increases the insulin sensitivity of body tissues), 2—glibenclamide (known since 1960s, stimulates insulin secretion by pancreatic β-cells, increases insulin release), 3—sitagliptin (DPP-4 inhibitor, Merck), 4—alogliptin (DPP-4 inhibitor, Takeda Pharmaceutical Company), 5—linagliptin (DPP-4 inhibitor, Boehringer Ingelheim), 6—tofogliflozin (SGLT-2 inhibitor, Chugai Pharma), 7—teneligliptin (DPP-4 inhibitor, Mitsubishi Tanabe Pharma), 8—rosiglitazone (PPAR agonist, GlaxoSmithKline), 9—pioglitazone (PPAR agonist).
2. Tetrazoles for Biomedicine
It can be seen from Figure 1 and the cited literature sources that most of the known antidiabetic drugs in use include nitrogen heterocyclic moieties: azoles, azines, annelated azoloazines, oxazoles, thiazoles, and some others. The development of novel antidiabetic agents often involves the use of polynitrogen heterocyclic systems with more than two endocyclic heteroatoms (triazoles, tetrazoles, oxadiazoles, or their fused derivatives) as key scaffolds [8][9][10][8,9,10]. These moieties are very promising in drug design because they are often metabolically stable, easily participate in various intermolecular interactions through hydrogen bonding, conjugation, or van der Waals bonding with biological targets, and many of them are bioisosters of functional groups of endogenous molecules [11].
Among other heterocyclic systems, the tetrazole cycle has a unique structure and unique properties. Despite the fact that the tetrazole cycle contains only one endogenous carbon atom and four nitrogen atoms, it is a very thermally and metabolically stable system. Previously, the synthesis and properties of tetrazoles have been discussed in detail in numerous reviews and monographs, including by the authors of this resviearchw [12][13][14][15][16][12,13,14,15,16].
Tetrazolyl moiety is present in the top 10 most frequent nitrogen heterocycles in US FDA-approved drugs and in a total of 16 pharmaceuticals [11]. This heterocycle is incorporated into active pharmaceutical ingredients of medications of various actions: hypotensives 10–12, diuretic 13, antihistamines 14–15, antibiotics, analgesics, and some others (Figure 2) [17].
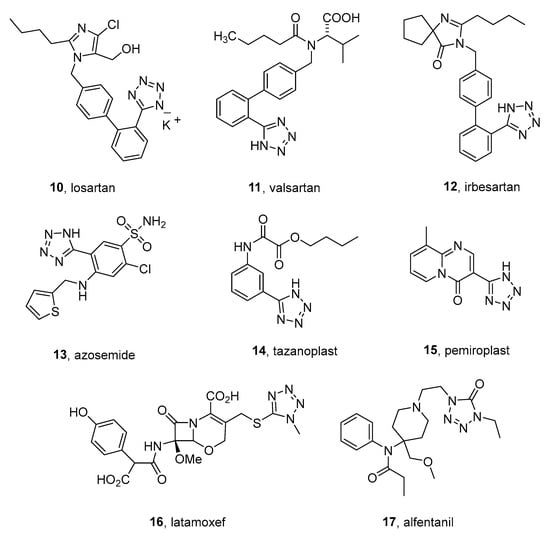
Figure 2. Some examples of tetrazole-containing medications.
Quite a lot of research has been devoted to the medicinal chemistry of tetrazoles, the results of which are summarized in a number of reviews on this topic [16][17][18][19][20][21][16,17,18,19,20,21]. Significant achievements have been made in the field of drug development for hypotensive, anticancer drugs, semi-synthetic antibiotics, and agents acting on the central nervous system [17][19][17,19]. However, there are few data points, and there is no systematic analysis of the antidiabetic activity of tetrazole derivatives. In view of the above, this review can fill this gap.
Tetrazoles vary greatly in properties depending on their prototropic form and substituent isomerism (Figure 3).
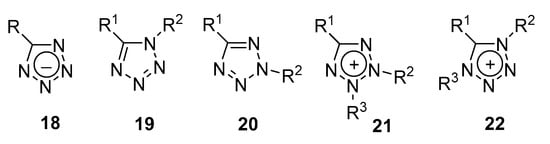
Figure 3. Main isomeric forms of tetrazoles.
Tetrazolate anions (tetrazolides) 18 are highly nucleophilic particles that are very soluble in water and other polar solvents and easily coordinate with transition metal ions [22]. Neutral 1H-tetrazoles 19 are usually more polar and thermally stable than 2H-tetrazoles 20. Isomeric tetrazolium ions 21, 22 are also very different in their properties. For example, cycle 21 can easily open under radiolysis, while ion 22 is still stable under these conditions [23]. The high stability of tetrazole forms can be partially explained with the sufficiently high aromaticity of the heterocycle. All the forms are planar and highly conjugated systems. Thus, the structural criteria of aromaticity for 2H-tetrazoles 20, especially tetrazolides 18, are close to those for benzene [24].
NH-Unsubstituted tetrazoles may exist as 1H- and 2H-tautomers 23 and 25 (Scheme 1). The most polar form 23 (where R is an electron-donor substituent or hydrogen) is preferred in condensed media (solutions or crystals), whereas the less polar 2H-form 24 with the same substituents prevails in the gas phase and non-polar solvents [25].
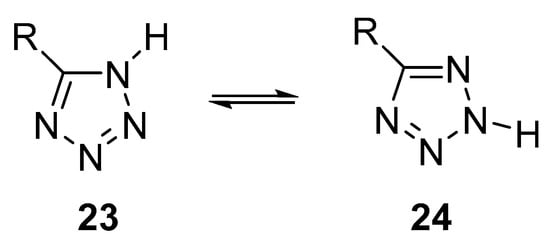
Scheme 1. Tautomerism of NH-unsubstituted tetrazoles.
Tetrazoles are relatively strong NH-acids and weak bases (Scheme 2). The acidity of NH-tetrazoles 23 is comparable to that of aliphatic carboxylic acids. The acidity of parent tetrazole is pKa 4.9, and depending on the nature of the C5-substituent, it can vary from 6 (for 5-aminotetrazole) to −1 (for 5-nitrotetrazole). Tetrazoles are also weak bases that ionize in strong mineral acids (for parent tetrazole, pKBH+ −2.7), and their basicity can vary in the range pKBH+ −1 ÷ −9 [25].
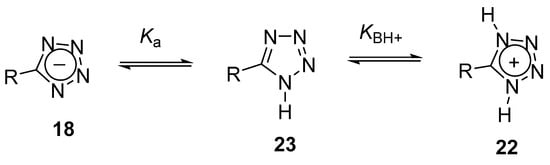
Scheme 2. Ionizations of tetrazoles.
A special mention should be made of the unique ability of the tetrazole cycle to form stable hydrogen bonds simultaneously with several proton donors and acceptors (Figure 4). Such interactions are often predominant in the binding of active molecules to their biological targets. BAs we have recently shown, based on theoretical and experimental pKHB values, tetrazoles are sufficiently strong bases for the formation of hydrogen bonding, and the substituent at position 5 of the cycle has a noticeable effect on the basicity [26].
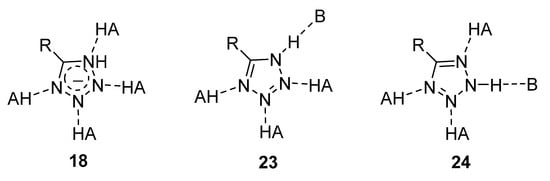
Figure 4. Possible ways of hydrogen bonding for different prototropic forms of 5-R-tetrazoles.
It is now generally accepted that neutral 1H-tetrazole forms 19, 23 are metabolically stable bioisosteric analogs of cis-amide and carboxyl groups, and tetrazolide 18 is an analog of carboxylate anion. However, in recent years, this concept of bioisosterism has been refined. As shown by Allen and co-authors, based on the analysis of crystallographic data and the results of theoretical calculations, the nature of hydrogen bond formation in pairs—1H-tetrazole-COOH and tetrazolide-carboxylate—is somewhat different [27]. The authors of the cited work also indicated that the functional groups of biomolecules linked by hydrogen bonds to 1H-tetrazole or tetrazolate anion are located at a distance greater by approximately 1.2 Å compared to the isosteric fragments of -COOH and -COO−. TheOur recent quantitative studies of the hydrogen bonding basicity of tetrazoles also support the fact that their ability to form hydrogen bonds is unique and significantly different from the carboxylic group [26].
