Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Elena A. Pudova and Version 2 by Rita Xu.
Chemotherapy based on taxane-class drugs is the gold standard for treating advanced stages of various oncological diseases. Drug resistance is the result of a combination of different events in the tumor cells under the influence of the drug, a comprehensive understanding of which has yet to be determined.
- prostate cancer
- chemoresistance
- docetaxel
- microRNAs
- lncRNAs
- circRNAs
1. Introduction
Prostate cancer (PCa) is a socially significant cancer and is the second most common type of cancer in men worldwide [1]. Most patients with PCa have localized disease and are treated with radical prostatectomy and/or radiation therapy followed by androgen deprivation therapy. However, within 10 years after androgen deprivation in patients, in 10–20% of cases, the disease develops into a prognostically unfavorable form—castration-resistant prostate cancer (CRPC), which is characterized by a significant deterioration in the quality of life and high mortality of patients. The median overall survival of patients with CRPC is less than 2 years [2]. Chemotherapy based on taxane-class drugs, such as paclitaxel and docetaxel, is the gold standard for the first-line therapy in this patient category. Docetaxel is the most commonly used first-line chemotherapy drug for CRPC. However, despite its widespread use in therapy, most patients eventually develop resistance, which is one of the reasons for the ineffectiveness of chemotherapy in patients [3].
Drug resistance is conventionally divided into two main classes: primary (existing initially) and acquired [4]. Primary resistance is characterized by the presence of various factors in tumor cells prior to the action of the drug, whereas acquired resistance represents a stepwise and slower process involving various molecular genetic and epigenetic events in the presence of the drug [5]. The mechanisms underlying acquired drug resistance are quite complex and involve alterations in the regulation of numerous genes and different signaling pathways, which act independently or in combination with other factors to inhibit the function of taxanes in tumor cells. Several studies have identified a range of mechanisms involved in the development of taxane resistance, yet the full extent of the picture is still to be determined [6].
For a long time, non-coding RNAs were considered as by-products of transcription with little biological significance, in contrast to messenger RNAs (mRNAs). However, since the development of approaches such as high-throughput sequencing and in-depth bioinformatic analyses, new types of RNAs that do not encode proteins, collectively known as non-coding RNAs (ncRNAs), have been discovered. Currently, the most studied types of “classic ncRNAs” are microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). It has been shown that these molecules participate in many signaling cascades, regulate various physiological processes, and also play a role in diseases. Moreover, many ncRNAs have been identified as tumor suppressors/oncogenic factors in various types of cancer, including prostate cancer, and they may also participate in the development of drug resistance [7].
2. MicroRNAs
MicroRNAs (miRNAs) are regulatory molecules with a length of 20–22 nucleotides that are formed from longer stem-loop structures and can bind to and inhibit mRNA. These molecules are transcribed as primary miRNAs (pri-miRNAs) and processed in the nucleus by the proteins Drosha and Dgcr8 into precursor miRNAs (pre-miRNAs). After export to the cytoplasm, pre-miRNAs are cleaved, forming a miRNA/miRNA duplex. Only one of the two miRNAs formed will exert its inhibitory function, while the other undergoes degradation. The total number of known human miRNAs is constantly expanding and currently includes 1917 precursors and 2654 mature molecules (miRBase, version 22.1). Many miRNAs are often suppressed in drug-resistant cells, but under normal cellular conditions, they help regulate signaling pathways that promote cell survival. The ability to avoid programmed cell death, apoptosis, is one of the key characteristics of tumor cells that ensures their survival. Tumor cells, like normal cells, also possess proteins from the BCL-2 family that regulate apoptosis. These include Bad and Bax, which initiate cascades that activate apoptosis, and Bcl-2, Bcl-XL, and Mcl-1, which inhibit the apoptosis process [6]. Several microRNAs (miRNAs) have been demonstrated through various studies to regulate the development of docetaxel resistance in PCa cells by enhancing cell survival and inhibiting apoptosis. These miRNAs include miR-143, the miR-200 family, miR-21, miR-129-5p, miR-27a, miR-27b, miR-34a, miR-183, miR-195, miR-223-3p, miR-323, and miR-4735-3p (Figure 1).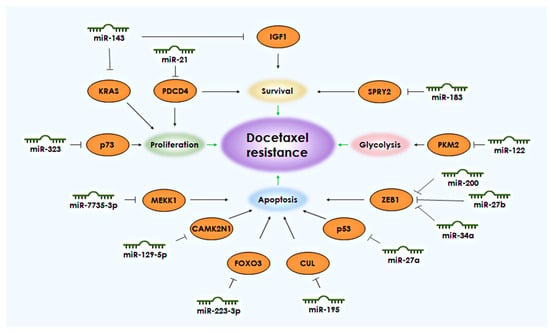
Figure 1. miRNAs and their targets associated with the development of resistance to docetaxel in PCa. The black arrows shows the connection between the target and the biological process. The green arrows indicate the connection of the process with the development of resistance to docetaxel.
3. LncRNAs
Long non-coding RNAs (lncRNAs) are regulatory molecules that are more than 200 nucleotides long and can be transcribed from introns, exons, intergenic regions, or non-coding regions. These molecules can perform various functions, such as participating in transcription as factors, acting as “sponges” in protein–protein interactions, but their most interesting action lies in inhibiting miRNAs. Specific lncRNAs can act as competing endogenous RNAs (ceRNAs), which sequester or inhibit microRNAs involved in pro-apoptotic pathways [20][26]. The exact number of known lncRNA genes is constantly increasing, and currently, the number of registered lncRNAs has reached 100,000 [21][27]. There are now hundreds of thousands of cataloged lncRNAs and dozens of databases with carefully curated information, such as LncATLAS, LncBook, LNCipedia, lncRNAKB, and others [22][28]. The upregulation of key lncRNAs and the subsequent suppression of corresponding miRNAs is also associated with taxane resistance in PCa. Based on various studies, a number of lncRNAs have been identified whose interaction with miRNAs is associated with the development of docetaxel resistance in PCa: NEAT1, UCA1, PCAT1, DANCR, CASC2, MALAT1, and LINC01963 (Figure 2).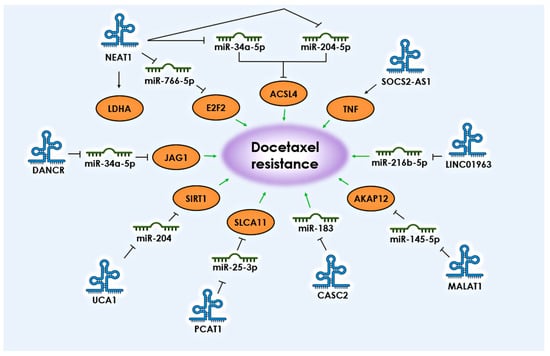
Figure 2. lncRNAs and their targets associated with the development of resistance to docetaxel in PCa. The black arrows shows the direction of communication between participants. Green arrows indicate the association with the development of docetaxel resistance.
