Lithium–sulfur (Li-S) batteries are considered one of the most promising energy storage systems due to their high theoretical capacity, high theoretical capacity density, and low cost. However, challenges such as poor conductivity of sulfur (S) elements in active materials, the “shuttle effect” caused by lithium polysulfide, and the growth of lithium dendrites impede the commercial development of Li-S batteries. As a crucial component of the battery, the separator plays a vital role in mitigating the shuttle effect caused by polysulfide. Traditional polypropylene, polyethylene, and polyimide separators are constrained by their inherent limitations, rendering them unsuitable for direct application in lithium–sulfur batteries. Therefore, there is an urgent need for the development of novel separators.
- lithium–sulfur batteries
- shuttle effect
- separator
- separator modification
1. Introduction
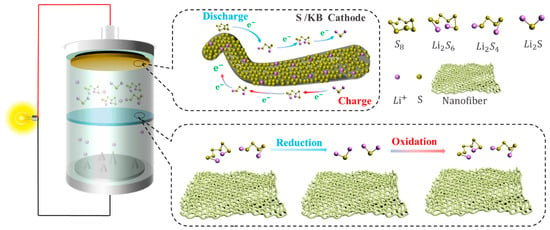
2. The Working Principle and Problems of Li-S
2.1. Working Mechanism of Li-S
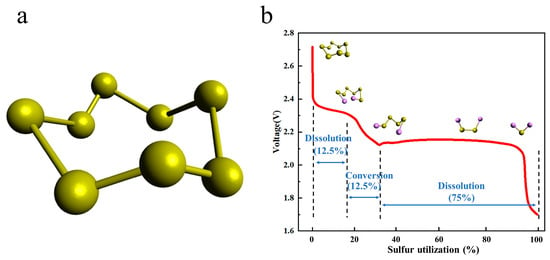
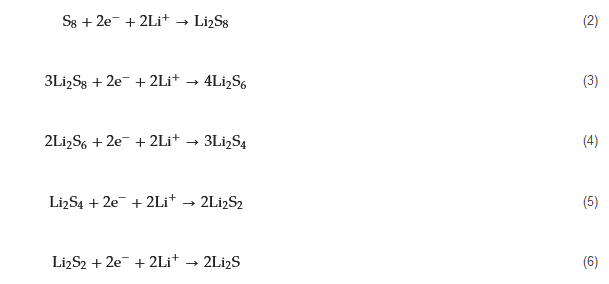

2.2. The Existing Problems of Li-S
2.2.1. Cathode
Because sulfur resources are abundant, non-toxic, and non-polluting; the theoretical specific capacity is 1675 mAh g−1; and they have low prices and other advantages, sulfur was selected as the cathode material in Li-S batteries [34,35,36][27][28][29]. However, problems such as low conductivity, volume expansion during electrochemical reactions, and shuttle effect lead to low coulombic efficiency, and the actual specific capacity is much lower than the theoretical specific capacity, in addition to poor cycle stability [37][30].2.2.2. Separator
As a vital part of Li-S, separators play a great role in the performance of Li-S [41][31]. During the charging and discharging process of lithium–sulfur batteries, the separator does not directly participate in the electrochemical reaction of mutual conversion between polysulfides [42][32]. However, the wettability, thermal stability, mechanical properties, porosity, liquid absorption rate, and other properties of the separator affect the specific capacity and cycle life of the Li-S. As a separator for Li-S, it is not only necessary to maintain the advantages of wettability, thermal stability, mechanical properties, porosity, and liquid absorption rate [43][33], but also to effectively suppress the shuttle effect [44][34].2.2.3. Anode
The weight density is low, and the theoretical specific capacity is 3860 mAh g−1 higher [45][35]. Due to its low reduction potential [46,47][36][37] and other advantages, lithium metal was chosen as the electrode material for Li-S. Metallic lithium is used as an anode in Li-S [48][38] due to the growth of lithium dendrites [40,49,50,51][39][40][41][42]. Lithium dendrites easily pierce the separator, resulting in potential safety hazards in the circuit. Lithium metal itself has strong activity and can easily have side reactions with electrolytes, resulting in low battery cycle life and other problems that limit the commercial development of Li-S.2.2.4. Electrolyte
Electrolytes, as a pivotal constituent bridging the positive and negative electrodes, wield substantial influence on the transport of lithium ions, thereby exerting a direct impact on the performance of lithium–sulfur batteries. Electrolytes can be classified into two categories: liquid electrolytes and solid electrolytes. Presently, liquid electrolytes find extensive application in lithium–sulfur batteries, primarily owing to their facile synthesis, high ionic conductivity, and favorable chemical stability. Nevertheless, liquid electrolytes are not without their challenges. Firstly, at the positive electrode, liquid electrolytes tend to dissolve polysulfides, which subsequently diffuse through the electrolyte to the negative electrode, leading to diminished Coulombic efficiency and corrosion of the lithium negative electrode. Secondly, liquid electrolytes typically comprise flammable organic solvents, thus posing safety concerns when exposed to elevated temperatures. In response to these issues associated with liquid electrolytes, a substantial body of researchers is actively engaged in addressing these challenges. This includes the modification of electrolyte composition, the exploration of novel multi-component solvents, and the incorporation of functional additives [58][43].2.2.5. Binders
Binders, constituting an integral part of the sulfur cathode in lithium–sulfur batteries, serve the crucial function of ensuring effective electrochemical contact among the conductive agent, sulfur, and current collector. Moreover, they mitigate volumetric variations of active materials during cycling. Binders play a pivotal role in lithium–sulfur batteries, and the commonly utilized types encompass polymeric, bio-based, and inorganic binders. Challenges regarding binders encompass inadequate mechanical properties and the detachment of electrode materials during cycling, consequently leading to a diminished cycling lifespan and reduced stability of the battery.2.2.6. Current Collector
Aluminum foil has found widespread application as a current collector for sulfur cathodes. However, at elevated temperatures, aluminum and sulfur can undergo reactions, which may pose safety concerns. Presently, the use of carbon-coated aluminum foil is employed to mitigate direct contact between sulfur and aluminum foil, thereby enhancing the safety of lithium–sulfur batteries. The presence of carbon coatings effectively improves the adhesion between the active materials and the current collector, while simultaneously enhancing electrical conductivity [60][44] (Figure 3).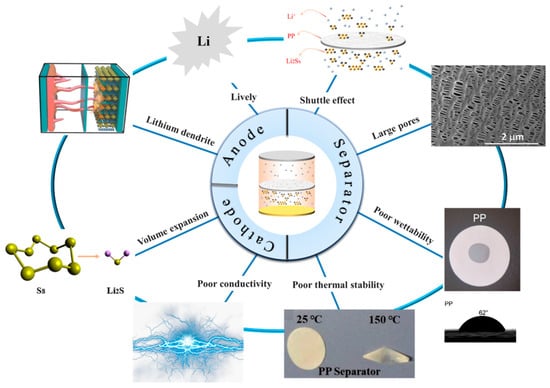
3. Methods
With the development of science and technology, separator technology has also been continuously developed and updated, and the methods of preparing polymer separators have also been diversified. Different preparation methods can be selected according to the material properties and separator application direction. The battery separator film is closely related to the energy density, stability, and cycle life of the battery [62][46]. A battery separator composed of nanofibers has the advantages of a large specific area and high porosity [63][47], which has attracted close attention. There are many methods of separators, but electrospinning [64][48], vacuum filtration [65][49], wet spinning [66][50], the coating method [67[51][52],68], the in situ growth method [69][53], and atomic layer deposition [70][54] are more commonly used to prepare separators.3.1. Electrospinning
Electrospinning is a novel technique for preparing nanofiber separators, the principle of which is as follows:: under the action of a high-voltage electrostatic field, the polymer forms a Taylor cone when it flows out of the needle, and a continuously charged jet is ejected and deposited on the collector as nonwoven nanofibers [71][55]. The nanofibers prepared by electrospinning can reach the nanometer diameter [64,72][48][56], and the prepared nanofiber separator has the advantages of high porosity, large specific surface area, and small pore size [73][57]. As shown in Figure 54, electrospinning technology has prepared a variety of nanostructured fibers. The electrospinning method has received great attention in the field of battery separators in recent years.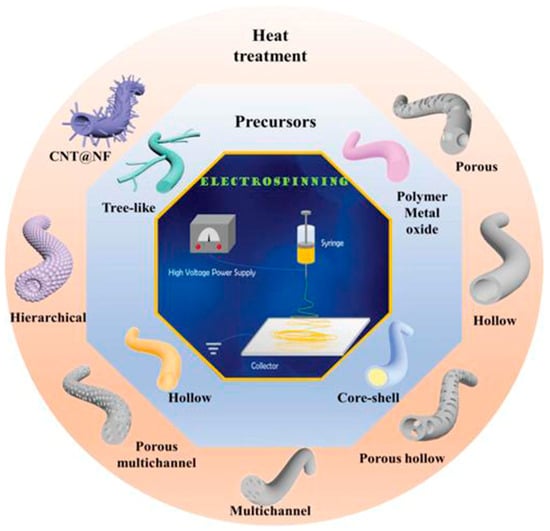
3.2. Vacuum Filtration
3.3. Wet Spinning
3.4. Coating Method
3.5. In Situ Growth Method
3.6. Atomic Layer Deposition (ALD)
4. Application of Separators in Lithium–Sulfur Batteries
4.1. Electrospinning
4.1.1. Separator
4.1.2. Interlayer
A highly conductive electrospun nanofiber interlayer is introduced between the positive electrode and the separator to effectively improve the performance of lithium–sulfur batteries. Electrospun carbon nanofibers, owing to their excellent electrical conductivity, serve to reduce internal resistance within batteries, thus enhancing the rates of electron and ion transport. Additionally, carbon nanofibers exhibit Van Der Waals interactions with polysulfides, facilitating their adsorption. Guo et al. [81][65]. utilized electrospinning technology to prepare a Ti4O7/C nanofiber (TCNF) interlayer. The incorporation of carbon nanofibers in this interlayer offered several advantages, including a large specific surface area and high electrical conductivity, which significantly enhanced the conversion and electron transfer of polysulfides. Additionally, Ti4O7 formed strong chemical bonds with polysulfides, effectively mitigating their shuttle effect.4.2. Vacuum Filtration
4.2.1. Separator
According to Yigeng et al. [89][66], They modified the PP separator through suction filtration, a depositing a layer of g-C3N4 composite on one side of it. It had abundant adsorption sites and contributed to the solidification of polysulfides. The process of polysulfide solidification entails immobilizing polysulfide species onto the cathode material or the cathode-proximate regions of the separator. This strategic immobilization serves as an effective countermeasure against the undesirable migration of polysulfides towards the anode, thereby mitigating capacity fade and consequentially augmenting the performance characteristics of lithium–sulfur batteries.4.2.2. Interlayer
Feng et al. [90][67] prepared a 2D NiCo MOF/CNT as the middle layer of a lithium–sulfur battery and filtered it onto PP through vacuum filtration. The thickness of 2D NiCo MOF/CNT was only a few nanometers, and the CNT built a conductive network to enhance electronic conductivity while serving as a physical barrier to prevent polysulfide migration. The 2D NiCo MOF/CNT improved the catalytic performance due to abundant and accessible active sites. The lithium–sulfur battery using 2D NiCo MOF/CNT interlayer had an initial discharge-specific capacity of 1132.7 mAh g−1 at 0.5 C, and it maintained 709.1 mAh/g-1 after 300 cycles, showing good cycle stability and rate performance.4.3. Wet Spinning
Currently, commercial PP and PE separators are prepared using a wet process. However, when used directly in lithium–sulfur batteries, the PP separator leads to a significant shuttle effect, resulting in a sharp decrease in the specific discharge capacity of the battery. Main applications include functional interlayers in Li-S [18,97,98,99,100][11][68][69][70][71] and electrodes [101,102][72][73]. Li et al. [98][69] successfully prepared a carbon paper sandwich via a wet process with excellent electrical conductivity of 11.9 S cm−1. A high initial capacity, up to 1091 mAh g−1, was achieved when using carbon paper as an interlayer for Li-S. At 5 C, 631 mAh g−1 was maintained after 200 cycles (0.21% capacity decay per cycle).4.4. Coating Method
4.4.1. Separator
Polar metal oxide titanium dioxide (TiO2) exhibits a strong chemical interaction with polysulfides, making it widely applicable in the field of lithium–sulfur batteries. This is attributed to the ability of oxygen atoms on the surface of TiO2 to form chemical bonds with sulfur atoms present in polysulfides, facilitating the effective adsorption of polysulfides. Additionally, the high electronegativity of the TiO2 surface enables it to counteract the shuttle effect of polysulfides through a charge repulsion mechanism. Gao et al. [57][74] coated the PP separator with a layer of titanium dioxide, modified multi-walled carbon nanotube composites (TiO2@SCNT/PP separator), and applied the separator to Li-S, and the data showed that the performance of the separator and the pre-modification period greatly improved.4.4.2. Interlayer
Wang et al. [104][75] prepared the NC-Co interlayer using a coating method. The intermediate layer effectively inhibited the shuttling of polysulfides. At 1 C, the first discharge-specific capacity of the lithium–sulfur battery using the interlayer was 1216.9 mAh g−1, and it maintained 660.3 mAh g−1 after 250 cycles. The Coulombic efficiency remained above 99% during the cycle. After 100 cycles, the surface SEM of the negative lithium metal showed that the lithium negative electrode with the interlayer had few surface cracks, while the lithium metal without the interlayer had obvious cracks, indicating that the use of the interlayer can significantly inhibit the corrosion of the negative metal lithium.4.5. In Situ Growth Method
4.5.1. Separator
Lu et al. [111][76] modified a PP separator via in situ growth. On the side of the PP separator, a layer of polar hydrated sulfate CoSO4·4H2O material (CS/PP separator) was grown in situ, and the cobalt sulfate hydrate had strong polarity and catalytic properties, which can effectively adsorb polysulfides. In the preparation flow chart, it can be seen by scanning electron microscopy that the surface of the separator was attached to a layer similar to the shape of a sea urchin, the single sea urchin was assembled from the nanoneedles of several microns, and the separator exhibited good mechanical stability. The data show that when the material reaction time was 6 h, the separator showed the best performance, which was denoted as CS/PP-6. At 1 C, the initial specific capacity of the modified separator reached as high as 807.7 mAh g−1. After 500 cycles, it still maintained 504.6 mAh g−1 (compared to 208.7 mAh g−1 for the PP separator), and the Coulombic efficiency reached as high as 97%. When the discharge current was restored to 0.1 C, the reversible capacity was 1308.6 mAh g−1, indicating that the lithium–sulfur battery using the separator had good reversibility and good electrochemical performance.4.5.2. Interlayer
Li et al. [112][77] prepared the ZIF/CNFs interlayer via the situ growth method. The interlayer had an obvious effect of inhibiting polysulfide shuttling. According to SEM and TEM images, ZIF-64 particles were distributed on the fiber, and due to the special binding site of ZIF-64, it inhibited the shuttling of polysulfides during circulation. At 1 C, it exhibited a high discharge-specific capacity of 1334 mAh/g, which remained at 569 mAh/g after 300 cycles.4.6. Atomic Layer Deposition
4.6.1. Separator
At present, owing to the distinctive attributes of the Atomic Layer Deposition (ALD) fabrication process, there is a scarcity of literature that directly employs ALD for the modification of polypropylene (PP) separators. Usually, people use methods such as cooperating with other preparation processes (coating, suction filtration, etc.) to modify the separator.4.6.2. Interlayer
Lin et al. [70][54]. prepared the CNT@SACo interlayer by atomic layer deposition and applied it to lithium–sulfur batteries. From the experimental results, the CNT@SACo interlayer exhibits catalytic activity catalyzes the conversion of polysulfides, and inhibits the shuttling of polysulfides. The lithium–sulfur battery with the CNT@SACo interlayer exhibited a high discharge specific capacity of 880 mAh/g at 1 C, and maintained a capacity of 595 mAh/g after 500 cycles, with a capacity decay rate of 0.064% per cycle.5. Conclusions
In recent years, with the rising demand for new energy, it has become important to develop an energy storage system with high energy density, low cost, and a long cycle life. Traditional lithium batteries have a high cost and low energy density, which makes it difficult for them to meet the huge market demand. Li-S batteries are regarded as one of the most promising energy storage systems due to their high theoretical specific capacity and low cost. Li-S batteries also have some urgent problems to solve, such as poor conductivity of S, the expansion of the positive electrode volume during the electrochemical reaction, and the most important problem, the shuttle effect caused by polysulfides. As an important component of lithium–sulfur batteries, separators are very important in suppressing the shuttle effect of polysulfides.References
- Kang, N.; Lin, Y.X.; Yang, L.; Lu, D.P.; Xiao, J.; Qi, Y.; Cai, M. Cathode porosity is a missing key parameter to optimize lithium-sulfur battery energy density. Nat. Commun. 2019, 10, 4597.
- Liu, M.; Deng, N.P.; Ju, J.G.; Fan, L.L.; Wang, L.Y.; Li, Z.J.; Zhao, H.J.; Yang, G.; Kang, W.M.; Yan, J.; et al. A Review: Electrospun Nanofiber Materials for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1905467.
- Din, M.M.U.; Murugan, R. Metal Coated Polypropylene Separator with Enhanced Surface Wettability for High Capacity Lithium Metal Batteries. Sci. Rep. 2019, 9, 16795.
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational Design of Nanostructured Functional Interlayer/Separator for Advanced Li-S Batteries. Adv. Funct. Mater. 2018, 28, 1707411.
- Cuisinier, M.; Hart, C.; Balasubramanian, M.; Garsuch, A.; Nazar, L.F. Radical or Not Radical: Revisiting Lithium-Sulfur Electrochemistry in Nonaqueous Electrolytes. Adv. Energy Mater. 2015, 5, 1401801.
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527.
- Song, Y.Z.; Cai, W.L.; Kong, L.; Cai, J.S.; Zhang, Q.; Sun, J.Y. Rationalizing Electrocatalysis of Li-S Chemistry by Mediator Design: Progress and Prospects. Adv. Energy Mater. 2020, 10, 1901075.
- Ren, W.C.; Ma, W.; Zhang, S.F.; Tang, B.T. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732.
- Liu, H.; Lai, W.-H.; Yang, H.-L.; Zhu, Y.-F.; Lei, Y.-J.; Zhao, L.; Peng, J.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K. Efficient separators with fast Li-ion transfer and high polysulfide entrapment for superior lithium-sulfur batteries. Chem. Eng. J. 2021, 408, 127348.
- Phung, J.; Zhang, X.Z.; Deng, W.J.; Li, G. An overview of MOF-based separators for lithium-sulfur batteries. Sustain. Mater. Technol. 2022, 31, e00374.
- Zhang, Y.S.; Zhang, X.L.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G.S. Lithium-Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 2103879.
- Zhang, Y.G.; Yang, S.; Zhou, S.Y.; Zhang, L.B.; Gu, B.B.; Dong, Y.Y.; Kong, S.Z.; Cai, D.; Fang, G.Y.; Nie, H.G.; et al. Oxygen doping in antimony sulfide nanosheets to facilitate catalytic conversion of polysulfides for lithium-sulfur batteries. Chem. Commun. 2021, 57, 3255–3258.
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134.
- Cuisinier, M.; Cabelguen, P.E.; Evers, S.; He, G.; Kolbeck, M.; Garsuch, A.; Bolin, T.; Balasubramanian, M.; Nazar, L.F. Sulfur Speciation in Li-S Batteries Determined by Operando X-ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 3227–3232.
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Analysis of Polysulfide Dissolved in Electrolyte in Discharge-Charge Process of Li-S Battery. J. Electrochem. Soc. 2012, 159, A421–A425.
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ. Sci. 2015, 8, 3477–3494.
- Zhao, E.Y.; Nie, K.H.; Yu, X.Q.; Hu, Y.S.; Wang, F.W.; Xiao, J.; Li, H.; Huang, X.J. Advanced Characterization Techniques in Promoting Mechanism Understanding for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707543.
- Sun, J.; Sun, Y.M.; Pasta, M.; Zhou, G.M.; Li, Y.Z.; Liu, W.; Xiong, F.; Cui, Y. Entrapment of Polysulfides by a Black-Phosphorus-Modified Separator for Lithium-Sulfur Batteries. Adv. Mater. 2016, 28, 9797–9803.
- Yao, S.S.; Cui, J.; Huang, J.Q.; Lu, Z.H.; Deng, Y.; Chong, W.G.; Wu, J.X.; Ihsan Ul Haq, M.; Ciucci, F.; Kim, J.K. Novel 2D Sb2S3 Nanosheet/CNT Coupling Layer for Exceptional Polysulfide Recycling Performance. Adv. Energy Mater. 2018, 8, 1800710.
- Park, J.; Kim, E.T.; Kim, C.; Pyun, J.; Jang, H.S.; Shin, J.; Choi, J.W.; Char, K.; Sung, Y.E. The Importance of Confined Sulfur Nanodomains and Adjoining Electron Conductive Pathways in Subreaction Regimes of Li-S Batteries. Adv. Energy Mater. 2017, 7, 1700074.
- Wu, J.Y.; Zeng, H.X.; Li, X.W.; Pei, H.J.; Xue, Z.G.; Ye, Y.S.; Xie, X.L. Dual-Functional Interlayer Based on Radially Oriented Ultrathin MoS2 Nanosheets for High-Performance Lithium Sulfur-Batteries. Acs Appl. Energy Mater. 2019, 2, 1702–1711.
- Zhao, M.; Li, B.-Q.; Chen, X.; Xie, J.; Yuan, H.; Huang, J.-Q. Redox Comediation with Organopolysulfides in Working Lithium-Sulfur Batteries. Chem 2020, 6, 3297–3311.
- Zhao, M.; Peng, Y.-Q.; Li, B.-Q.; Zhang, X.-Q.; Huang, J.-Q. Regulation of carbon distribution to construct high-sulfur-content cathode in lithium–sulfur batteries. J. Energy Chem. 2021, 56, 203–208.
- Sadd, M.; De Angelis, S.; Colding-Jorgensen, S.; Blanchard, D.; Johnsen, R.E.; Sanna, S.; Borisova, E.; Matic, A.; Bowen, J.R. Visualization of Dissolution-Precipitation Processes in Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103126.
- Ren, Y.X.; Zhao, T.S.; Liu, M.; Tan, P.; Zeng, Y.K. Modeling of lithium-sulfur batteries incorporating the effect of Li2S precipitation. J. Power Sources 2016, 336, 115–125.
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908.
- Guo, J.; Yang, Z.; Yu, Y.; Abruna, H.D.; Archer, L.A. Lithium-sulfur battery cathode enabled by lithium-nitrile interaction. J. Am. Chem. Soc. 2013, 135, 763–767.
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647.
- Barai, P.; Mistry, A.; Mukherjee, P.P. Poromechanical effect in the lithium–sulfur battery cathode. Extrem. Mech. Lett. 2016, 9, 359–370.
- Yan, J.H.; Liu, X.B.; Li, B.Y. Capacity Fade Analysis of Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1600101.
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, N.A.; Liang, B.; Iqbal, A.; Wang, J.X.; Sin, H.S.; Li, L.S.; Tang, Z.Y. MoS2/Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1606817.
- Zhai, P.; Liu, K.; Wang, Z.; Shi, L.; Yuan, S. Multifunctional separators for high-performance lithium-ion batteries. J. Power Sources 2021, 499, 229973.
- Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; He, W.; et al. Inhibiting Polysulfide Shuttling with a Graphene Composite Separator for Highly Robust Lithium-Sulfur Batteries. Joule 2018, 2, 2091–2104.
- Gao, Z.; Xue, Z.; Miao, Y.; Chen, B.; Xu, J.; Shi, H.; Tang, T.; Zhao, X. TiO2@Porous carbon nanotubes modified separator as polysulfide barrier for lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164249.
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.-G. Anodes for Rechargeable Lithium-Sulfur Batteries. Adv. Energy Mater. 2015, 5, 1402273.
- Cheng, X.B.; Huang, J.Q.; Zhang, Q. Review-Li Metal Anode in Working Lithium-Sulfur Batteries. J. Electrochem. Soc. 2018, 165, A6058–A6072.
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Characterization of the solid electrolyte interphase on lithium anode for preventing the shuttle mechanism in lithium–sulfur batteries. J. Power Sources 2014, 246, 840–845.
- Rong, G.L.; Zhang, X.Y.; Zhao, W.; Qiu, Y.C.; Liu, M.N.; Ye, F.M.; Xu, Y.; Chen, J.F.; Hou, Y.; Li, W.F.; et al. Liquid-Phase Electrochemical Scanning Electron Microscopy for In Situ Investigation of Lithium Dendrite Growth and Dissolution. Adv. Mater. 2017, 29, 1606187.
- Wang, J.; Yi, S.; Liu, J.; Sun, S.; Liu, Y.; Yang, D.; Xi, K.; Gao, G.; Abdelkader, A.; Yan, W.; et al. Suppressing the Shuttle Effect and Dendrite Growth in Lithium-Sulfur Batteries. ACS Nano 2020, 14, 9819–9831.
- Rosso, M.; Brissot, C.; Teyssot, A.; Dolle, M.; Sannier, L.; Tarascon, J.M.; Bouchetc, R.; Lascaud, S. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 2006, 51, 5334–5340.
- Fan, L.; Chen, S.H.; Zhu, J.Y.; Ma, R.F.; Li, S.P.; Podila, R.; Rao, A.M.; Yang, G.Z.; Wang, C.X.; Liu, Q.; et al. Simultaneous Suppression of the Dendrite Formation and Shuttle Effect in a Lithium-Sulfur Battery by Bilateral Solid Electrolyte Interface. Adv. Sci. 2018, 5, 1700934.
- Hu, Y.; Chen, W.; Lei, T.; Jiao, Y.; Wang, H.; Wang, X.; Rao, G.; Wang, X.; Chen, B.; Xiong, J. Graphene quantum dots as the nucleation sites and interfacial regulator to suppress lithium dendrites for high-loading lithium-sulfur battery. Nano Energy 2020, 68, 104373.
- Wang, L.; Liu, J.; Yuan, S.; Wang, Y.; Xia, Y. To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 2016, 9, 224–231.
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162.
- Li, Y.; Wang, X.; Liang, J.; Wu, K.; Xu, L.; Wang, J. Design of a high performance zeolite/polyimide composite separator for lithium-ion batteries. Polymers 2020, 12, 764.
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262.
- Zhao, M.; Wang, J.; Chong, C.B.; Yu, X.W.; Wanga, L.L.; Shi, Z.Q. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120.
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391.
- Gao, W.L.; Kono, J. Science and applications of wafer-scale crystalline carbon nanotube films prepared through controlled vacuum filtration. R. Soc. Open Sci. 2019, 6, 181605.
- Kritzer, P. Nonwoven support material for improved separators in Li–polymer batteries. J. Power Sources 2006, 161, 1335–1340.
- Zhu, J.; Ge, Y.; Kim, D.; Lu, Y.; Chen, C.; Jiang, M.; Zhang, X. A novel separator coated by carbon for achieving exceptional high performance lithium-sulfur batteries. Nano Energy 2016, 20, 176–184.
- Shao, H.; Wang, W.; Zhang, H.; Wang, A.; Chen, X.; Huang, Y. Nano-TiO2 decorated carbon coating on the separator to physically and chemically suppress the shuttle effect for lithium-sulfur battery. J. Power Sources 2018, 378, 537–545.
- Yang, L.W.; Wang, Y.; Li, Q.; Li, Y.; Chen, Y.X.; Liu, Y.X.; Wu, Z.G.; Wang, G.K.; Zhong, B.H.; Song, Y.; et al. Inhibition of the shuttle effect of lithium-sulfur batteries via a tannic acid-metal one-step in situ chemical film-forming modified separator. Nanoscale 2021, 13, 5058–5068.
- Lin, Q.Y.; Ding, B.; Chen, S.; Li, P.; Li, Z.W.; Shi, Y.Y.; Dou, H.; Zhang, X.G. Atomic Layer Deposition of Single Atomic Cobalt as a Catalytic Interlayer for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 11206–11212.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347.
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309.
- Hao, J.; Lei, G.; Li, Z.; Wu, L.; Xiao, Q.; Wang, L. A novel polyethylene terephthalate nonwoven separator based on electrospinning technique for lithium ion battery. J. Membr. Sci. 2013, 428, 11–16.
- Zhang, L.Y.; Batchelor, W.; Varanasi, S.; Tsuzuki, T.; Wang, X.G. Effect of cellulose nanofiber dimensions on sheet forming through filtration. Cellulose 2012, 19, 561–574.
- Cho, T.-H.; Tanaka, M.; Ohnishi, H.; Kondo, Y.; Yoshikazu, M.; Nakamura, T.; Sakai, T. Composite nonwoven separator for lithium-ion battery: Development and characterization. J. Power Sources 2010, 195, 4272–4277.
- Safavi, A.; Fathi, S.; Babaei, M.R.; Mansoori, Z.; Latifi, M. Experimental and numerical analysis of fiber characteristics effects on fiber dispersion for wet-laid nonwoven. Fibers Polym. 2009, 10, 231–236.
- Wang, X.R.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904.
- Yan, B.; Li, X.; Bai, Z.; Song, X.; Xiong, D.; Zhao, M.; Li, D.; Lu, S. A review of atomic layer deposition providing high performance lithium sulfur batteries. J. Power Sources 2017, 338, 34–48.
- Zhu, X.B.; Ouyang, Y.; Chen, J.W.; Zhu, X.G.; Luo, X.; Lai, F.L.; Zhang, H.; Miao, Y.E.; Liu, T.X. In situ extracted poly(acrylic acid) contributing to electrospun nanofiber separators with precisely tuned pore structures for ultra-stable lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 3253–3263.
- Guo, P.; Jiang, P.; Chen, W.; Qian, G.; He, D.; Lu, X. Bifunctional Al2O3/polyacrylonitrile membrane to suppress the growth of lithium dendrites and shuttling of polysulfides in lithium-sulfur batteries. Electrochim. Acta 2022, 428, 140955.
- Guo, Y.; Li, J.; Pitcheri, R.; Zhu, J.; Wen, P.; Qiu, Y. Electrospun Ti4O7/C conductive nanofibers as interlayer for lithium-sulfur batteries with ultra long cycle life and high-rate capability. Chem. Eng. J. 2019, 355, 390–398.
- Huangfu, Y.; Zheng, T.; Zhang, K.; She, X.; Xu, H.; Fang, Z.; Xie, K. Facile fabrication of permselective g-C3N4 separator for improved lithium-sulfur batteries. Electrochim. Acta 2018, 272, 60–67.
- Feng, P.; Hou, W.; Bai, Z.; Bai, Y.; Sun, K.; Wang, Z. Ultrathin two-dimensional bimetal NiCo-based MOF nanosheets as ultralight interlayer in lithium-sulfur batteries. Chin. Chem. Lett. 2023, 34, 107427.
- Meng, L.; Li, Y.; Lin, Q.X.; Long, J.; Wang, Y.; Hu, J. Nitrogen and Oxygen Dual Self-Doped Flexible PPTA Nanofiber Carbon Paper as an Effective Interlayer for Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2021, 4, 8592–8603.
- Li, Y.; Meng, L.; Jin, L.; Yun, L.; Jian, H. A wet-laid carbon paper with 3D conductive structure as an interlayer for lithium-sulfur batteries. Mater. Res. Express 2019, 6, 125547.
- Chong, W.G.; Xiao, F.; Yao, S.S.; Cui, J.; Sadighi, Z.; Wu, J.X.; Ihsan-Ul-Haq, M.; Shao, M.H.; Kim, J.K. Nitrogen-doped graphene fiber webs for multi-battery energy storage. Nanoscale 2019, 11, 6334–6342.
- Suriyakumar, S.; Stephan, A.M. Mitigation of Polysulfide Shuttling by Interlayer/Permselective Separators in Lithium-Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 8095–8129.
- Chong, W.G.; Huang, J.Q.; Xu, Z.L.; Qin, X.Y.; Wang, X.Y.; Kim, J.K. Lithium-Sulfur Battery Cable Made from Ultralight, Flexible Graphene/Carbon Nanotube/Sulfur Composite Fibers. Adv. Funct. Mater. 2017, 27, 1604815.
- Chong, W.G.; Xiao, Y.; Huang, J.-Q.; Yao, S.; Cui, J.; Qin, L.; Gao, C.; Kim, J.-K. Highly conductive porous graphene/sulfur composite ribbon electrodes for flexible lithium-sulfur batteries. Nanoscale 2018, 10, 21132–21141.
- Bruckner, J.; Thieme, S.; Bottger-Hiller, F.; Bauer, I.; Grossmann, H.T.; Strubel, P.; Althues, H.; Spange, S.; Kaskel, S. Carbon- Based Anodes for Lithium Sulfur Full Cells with High Cycle Stability. Adv. Funct. Mater. 2014, 24, 1284–1289.
- Wang, J.; Wu, T.; Zhang, S.; Gu, S.; Jin, J.; Wen, Z. Metal-organic-framework-derived N-C-Co film as a shuttle-suppressing interlayer for lithium sulfur battery. Chem. Eng. J. 2018, 334, 2356–2362.
- Lu, X.; Wang, H.; Liu, X.; Song, Z.; Jiang, N.; Xie, F.; Zheng, Q.; Lin, D. Functional separators prepared via in-situ growth of hollow CoSO4 hydrate arrays on pristine polypropylene membrane for high performance lithium-Sulfur batteries. J. Alloys Compd. 2020, 838, 155618.
- Li, J.; Jiao, C.; Zhu, J.; Zhong, L.; Kang, T.; Aslam, S.; Wang, J.; Zhao, S.; Qiu, Y. Hybrid co-based MOF nanoboxes/CNFs interlayer as microreactors for polysulfides-trapping in lithium-sulfur batteries. J. Energy Chem. 2021, 57, 469–476.
