Humification (HF) is the natural process of converting bioorganic matter into humic substances (humus, humate, humic acid, fulvic acid, and humin) via geo-microbiological mechanisms under aerobic and/or anaerobic conditions. Humic substances (HSs) and their composition and concentrations mostly determine the basic properties of soils and play an important role in regulating the growth of plants and soil microorganisms and the accumulation and migration of metal ions, radionuclides, and ecotoxicants in soils. Various processes designed for the humification (HF) of animal husbandry wastes, primarily bird droppings, reduce their volumes, solve environmental problems, and make it possible to obtain products with artificially formed humic substances (HSs) as analogues of natural HSs, usually extracted from fossil sources (coal and peat).
- humification (HF)
- composting
- anaerobic digestion
- hydrothermal carbonation (HTC)
- pyrolysis
- alkaline hydrolysis
- animal wastes
1. Introduction
| Country/Reference | Animal Wastes [References] | AP * |
|---|---|---|
| USA | Dairy manure [11] | 24,000 |
| China | Livestock manure [12] | 3800 |
| Chicken manure [13] | 155.0 | |
| Brazil | Cattle manure [14] | 1900 |
| EU | Farm manure [15] | 1200 |
| France | Farm manure [16] | 214.3 |
| Germany | Farm manure [16] | 175.7 |
| The United Kingdom | Farm manure [16] | 112.0 |
| Spain | Farm manure [16] | 108.3 |
| Bangladesh | Cow manure [17] | 102.6 |
| Poland | Farm manure [16] | 91.3 |
| Italy | Farm manure [16] | 89.4 |
| India | Poultry manure [18] | 38.0 |
| Malaysia | Chicken manure [19] | 23.1 |
| Serbia | Farm manure [16] | 18.6 |
| Greece | Farm manure [16] | 16.9 |
| Belgorod Region, Russia | Total manure [20] | 14.2 |
| Turkey | Chicken manure [21] | 11.0 |
| Canary Islands | Livestock manure [22] | 0.5 |
| Malta | Farm manure [16] | 0.3 |
| South Africa | Cattle manure [23] | 0.1 |
| Substrate (References) | Conditions/Additives | Products |
|---|---|---|
| Composting | ||
| Dairy manure [28] | Thermal pretreatment (90 °C, 4 h), 60 days | Compost with 75.0–77.0 g of HS/kg |
| Cow dung and corn straw (ratio of 1:2) [29] | Addition of 2.5–5% (d.w.) FeSO4, 50 days | Compost with 109.8–129.9 g of HS/kg |
| Maize straw and chicken manure (ratio of 6:1) [30] | Addition of benzoic acid (5% d.w.) and soybean residue after oil extraction (15% d.w.), 62 days | Compost with 150.0 g of HS/kg |
| Dairy manure and sugarcane leaves and (ratio of 4:1) [31] | Two-step inoculation (0 and 9 days) by Bacillus licheniformis, Aspergillus nidulans and A. oryzae cells (ratio of 1:1:1 w/w/w)—2% d.w., 45 days | Compost with 70.0 g of HS/kg |
| Fresh dairy manure and sawdust (ratio of 3.5:1) [32] | Treatment with 0.2 M of H2O2 (0.5 L) and CuCl2 (0.5 g/kg of compost), 46 days | Compost with 151.9 g of HS/kg |
| Pig manure and sawdust (ratio of 2:1) [33] | Addition of Black Tourmaline—10% d.w., 42 days | Compost with 50.2 g HA/kg and 24.0 g FA/kg |
| Dairy manure and bagasse pith (ratio of 3:1) [34] | Addition of H2O2 (2.14 mmol/kg) and ascorbic acid (3.57 mmol/kg of the d.w.), 34 days | Compost with 180.0 g of HS/kg |
| Chicken manure and rice husk (ratio of 6.7:1) [35] | Hyper thermophilic pretreatment (≥80 °C) for 1–9 days and total process for 44 days | Compost with 65% HS of TS (according to calculations ~260 g of HS/kg) |
| Pig manure and rice straw (C/N = 25) [36] | Hyper thermophilic pretreatment (90 °C, 4 h), 60 days | Compost with 87.8 g of HS/kg |
| Chicken manure and corn straw (C/N = 20) [37] | Addition of malonic acid (0.5%), MnO2 (0.5% d.w.), or their combination, 60 days | Compost with 75.0–87.0 g of HS/kg |
| Chicken manure, sawdust and urea (C/N = 30) [38] | Addition of 0.1% adenosine triphosphate or 0.5% malonic acid (d.w.), 49 days | Compost with 40.0–50.0 g of HS/kg |
| Digestates and chicken manure [39] | Without additives, 60 days | Compost with 90.0–95.0 g of HS/kg |
| Swine manure and corn stalk (ratio of 6:1) [40] | Addition of 1.0% (v/w) Acinetobacter pittii, Bacillus subtilis, B. altitudinis (ratio of 1:2:1 v/v), 32 days | Compost with 88.1 g of HS/kg |
| Cattle manure (6.7–30% dry basis), rice straw (21.7–31.7%), biogas residue (30–70%), food waste (8.3%) [12] | Without additives, 30 days | Compost with 75.0–88.5 g of HS/kg |
| Dairy manure and bagasse [41] | Addition of 10% Red mud (d.w.), pH 8.7, 45 days | Compost with 115.0–120.0 g of HS/kg |
| Cow manure and sugar cane straw (ratio of 5:1) [42] | Addition of 5% biochar from wood obtained via high-temperature gasification (400–550 °C), 40 days | Compost with 29.0–31.0 g of HS/kg |
| Chicken manure and rice hulls (C/N = 25) [43] | Addition of lignite (15% w/w), 55 days | Compost with 80.2 g of HS/kg |
| Chicken manure and rice straw (C/N =25–30) [44] | Addition of 7.5% montmorillonite (w/w) and pretreatment at 550 °C, 60 days | Compost with 67.0–71 g of HS/kg |
| Chicken manure and spent mushroom substrate (ratio of 1:1.2) [45] | Addition of Garden waste (15% fresh weight), 60 days | Compost with 145.0–155.0 g of HS/kg |
| Horse manure (C/N = 33) [46] | Vermicomposting (10 g of earthworms (Eisenia andrei)/kg), 35 °C, 6–9 months | Compost with 26.0–26.6 g of HA/kg |
| Anaerobic digestion | ||
| Chicken manure [47] | 37 °C, 10.0% of TS, and 7.9% VS, 40 days | Digestate—relative content of HLC (34%) and FLC (6%). HS yield was not controlled |
| Chicken manure [48] | 37 °C, 10.0% TS, and 7.9% VS, 25 days | Digestate—7.7 g HA/L |
| Turkey manure [49] | 37 °C, 51.2% (w/w wet basis) TS, and 71.5% (w/w dry basis) VS, OLR—0.5–2.5 kg VS/m3 per day, 77 days | Content HS in liquid fraction of the effluent and entire effluent (with digestate)—2.36 (2.32 HA, 0.04 FA) and 2.6 (2.04 HA, 0.60 FA) g/L |
| Sheep bedding and cattle manure [50] | 18 ± 4 °C; sheep-bedding-to-cattle-manure ratios of 0:100, 25:75, 50:50, 75:25, and 100:0; final content of TS –5%; 5 months | Digestate with HA/FA—1.3–3.0. HS yield was not controlled |
| Pig manure [51] | Hydrothermal pretreatment (70–170 °C, 0.5 h), 37 °C, 30 days | Digestate with HLC and FLC in amounts of 58.0–65.9 and 35.5–42.0%, respectively. HS yield was not controlled. |
| Hydrothermal carbonization | ||
| Dried swine manure [52] | 180 °C, 1 MPa, 15wt.% CaO, 10 h | HCmy—75.2% |
| Dried poultry litter [53] | 180 °C, 1 MPa, 1 h | HCmy—60.4% |
| Dried poultry litter [54] | 250 °C, 4–5 MPa, H2SO4 (pH 2.0), 2 h | HCmy—38.1% |
| Dry swine and chicken manure [55] | 240 °C, 3–4MPa, 10 h | HCmy—54.6% |
| Dried swine manure with cellulose [56] | 210 °C, 2 MPa, 5 h | HCmy—52.0% |
| Dried swine manure with sawdust [57] | 220 °C, 2–3 MPa, 10 h | HCmy—61.8% |
| Dried pig manure [58] | 180 °C, 1 MPa, 1–1.5 g KOH per 100 g manure, 1 h | HCmy—79.0% |
| Dried swine manure [59] | 200 °C, 2 MPa, 30 min | HCmy—58.7% |
| Chicken litter [60] | 220 °C, 2–3 MPa, 20 min | HCmy—68.0% |
| Air-dried pig manure [61] | 200 °C, 2 MPa, 2 h | HCmy—58.8% |
| Poultry and swine manure; dairy and beef cattle manure; broiler and layer chicken litter [62] | 180 °C, 1 MPa, 1 h | HCmy—67.3% |
| Mixture of chicken manure with sawdust [63] | 260 °C, 40 min | Biochar yield—95.1% |
| Dewatered poultry sludge [64] | 268 °C, 47 min | Biochar yield—85.0% |
| Pyrolysis | ||
| Dried pig manure [58] | 200 °C, 1 h | Biochar yield—40.0% |
| Poultry litter [65] | Wet torrefaction pretreatment (300 °C), 600 or 800 °C, supercritical CO2, 1.5–2 h | Biochar yield—51.2% |
| Chicken litter [60] | 400 °C, 20 min | Biochar yield—38.0% |
| Pre-dried broiler manure [66] | 350 °C, 1 h | Biochar yield—47.0% |
| Dried poultry litter [67] | 500 °C, Mixed with H3PO4 and MgO (biomass:H3PO4 ratio = 1:0.5 (w/w), molar P:Mg ratio—1:1), 2 h | Biochar yield—60.0% |
| Air-dried pig manure [61] | 300 °C, 1 h | Biochar yield—84.0% |
| Dried digested cattle manure [68] | 600 °C, 30 min | Biochar yield—44.8% |
| Poultry and swine manure, dairy and beef cattle manure, broiler and layer chicken litter [62] | 400 °C, 1 h | Biochar yield—51.0% |
| Dried goat manure [69] | 300 °C, 30 min | Biochar yield—48.6% |
| Air-dried poultry manure [70] | 200 °C, 4 h | Biochar yield—95.8% |
| Hydrolysis | ||
| Air-dried sheep or cow manures [71] | Acid hydrolysis (0.1–1.0 N HCl or H2SO4) at 105 °C and extraction (1N KOH), 1 h | HS yield—45 g/kg (sheep waste) and 56 g/kg (cow waste) |
| Air-dried poultry manure [72] | 25 °C, 0.1 N NaOH, 24 h | HA—28.1 g/kg. FA—13.3 g/kg |
| Farmyard manure [73] | 25 °C, 0.1 M NaOH, 450 rpm, 48 h | HA yield—10% |
| Fresh chicken manure [74] | Subcritical water extraction (230–250 °C, 6 MPa) | Liquid phase with 31.0 g of HA/kg and 20 g of FA/kg |
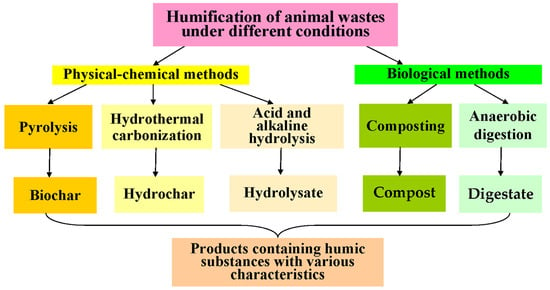
2. HF via Composting of AW
Composting with the formation of compost (Figure 1) containing different concentrations of HSs (26–260 g/kg) as the main product is widely used among the biological methods for the HF of AW (Table 2). Composting is an aerobic process during which the microbiological degradation of bioorganic substances occurs along with the formation of HS precursors (amino acids, reducing sugars, peptides, etc.) with their subsequent stabilization in self-forming supramolecular ensembles [76]. Composting consists of several stages: heating, cooling, and maturation. The temperature in the composted mass begins to rise a few hours after the beginning of the process as a result of biochemical degradation reactions with an increase in the concentrations of reducing sugars, nucleic and amino acids, phenol residues (in the case of the conversion of lignin-containing compounds present in animal excrement), etc. The subsequent decrease in the concentrations of these compounds occurs due to the formation of structurally complex HSs at the compost maturation stage [36]. The formation of HSs during composting is influenced by many factors (temperature, pH, C/N ratio, humidity, oxygen concentration, etc.). The maturity of compost is determined precisely by the change in the HS concentrations in its content [28][31][33][35][45]. An increase in the composting temperature ameliorates the destruction depth of organic compounds and promotes their interaction with the formation of HSs. The dynamics of increasing HS concentrations in compost also depend on temperature. The concentration of HA remains relatively constant during the first 20 days of the process and then gradually increases under mesophilic composting conditions [28]. With an increase in the composting temperature (up to 90 °C), the HS concentrations decrease by 25% in the first 30 days and then rise sharply as a result of the formation of HSs concurrently with the cooling and maturation of the compost [38][40][43]. There are general trends in the simultaneous increase in HA content and decrease in FA concentrations in the HS composition during mature compost formation [35][43]. FAs can be used as substrates by microorganisms and converted into HA via condensation and polymerization reactions, which lead to an increase in the aromaticity of HSs and an increase in HF. The HF index is considered to rise as the ratio of HA/FA increases [30]. The HA/FA ratio can reach 5.4–7.6, as a rule, after 1.5 months of composting in the final compost of AW [43]. The average HS concentration can reach 90–100 g/kg in mature compost with a predominance of high-molecular HAs in the HS composition, although the overall HS concentrations can vary in the compost due to the different initial characteristics of the treated AW (Table 2). The sequence of transformations of various functional groups in HS formation in a compost was analyzed using two-dimensional correlation spectroscopy (2D-COS) [34][35]. First, the C–O stretching of aromatic acid and aliphatic acid esters was observed. Then, the C–H deformation, vibration, and C–O stretching of polysaccharides or polysaccharide-like substances was observed. Further, the C=O stretching of carboxylate, quinone, ketone, or amide was witnessed. Finally, the C=C stretching of aromatic rings was confirmed [34]. The elemental composition of HSs during the composting of AW under hyper thermophilic conditions showed an increase in N-content, which is associated with enhanced polymerization and condensation of polysaccharide-like substances with N-containing compounds (proteins, nucleic acids, and amino acids) [35]. Composting is a long process: it can span from 1 to 2 months (Table 2) to 6 months or longer (with vermicomposting) [46]. Composting is characterized by low process speeds (Figure 2) in spite of high yields of conversion of organic substances into HSs. This is the reason why alternative methods for the HF of AW are being developed, leading to the production of HSs and the search for factors capable of HF acceleration.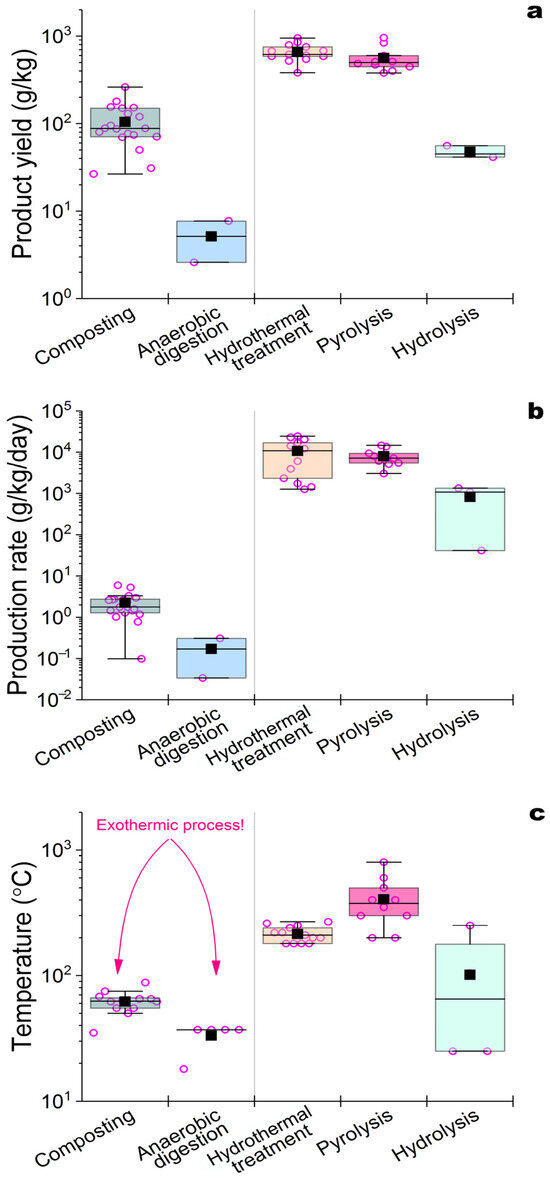
3. HF via Anaerobic Digestion of AW
Another biological method of converting AW into a product containing HSs is AD (Table 2, Figure 1). This process allows one to simultaneously obtain biogas—consisting mainly of CH4 and CO2, used as an alternative energy source—and digestate (Figure 1), containing a consortium of methanogenic cells, products of their metabolism, and HS-containing products of the anaerobic degradation of AW. Another composition of microorganisms involved in AD [77], in comparison with composting, determines other rates of HS accumulation in the resulting digestate (Table 2, Figure 2). Due to the action of the microbial consortia, some of the substances initially present in AW do not undergo destruction and conversion or undergo these processes extremely slowly under AD conditions. This applies to the lignocellulose components of processed excrements [78] and affects the characteristics of AD and the resulting product with HSs. The presence of organic N-containing compounds in the treated masses is another problem in the HF of AW via AD as compared to composting. To reduce the effect of N-containing compounds (in particular, urea) on AD, their membrane separation from the reaction medium is considered during the process [21]. For the successful conversion of AW into digestate containing HSs, the control of a larger number of factors in comparison with composting is required. The initial content of AW loaded in the AD reactor, the current pH value of the fermentation medium, temperature, the concentrations of the products formed, pressure created by accumulated gases in the working AD reactor, etc., should be controlled [25]. As in composting, with an increasing duration of the process, the concentration of humic-like compounds (HLC) gradually increases, while, on the contrary, the concentration of fulvic-like compounds (FLC) decreases [47]. These are common trends in changes in the composition of HSs in products obtained in two different biological processes for the HF of AW (composting and AD). The use of 2D-COS [48], as in the case of composting, made it possible to study the structural changes in the active functional groups of HSs obtained as a result of the AD of chicken manure. It was found that the active functional groups of HSs changed in the following sequence: aliphatic-like substances (C–H), amides (H–N) or carbohydrates (O–H), carboxylic acids (C=O), polysaccharides (C=O), aromatic compounds, and ketones (C=C). If we compare these results with those mentioned earlier for the HF of AW via composting (Section 2.1), then we can note a clear general similarity in the identified sequences of changes in organic matter after its HF under aerobic and anaerobic conditions. During the HF of lignocellulose raw materials, many more HLC are formed in AD than in the similar AD of AW. Lignocellulose provides an increased number of precursors for the formation of HSs, although the HF of plant raw materials under AD conditions is much slower [47]. It has been established that HSs formed with a more complex and stable structure [49][50] from lignocellulose are characterized by a high degree of aromaticity and have a notable inhibitory effect on AD. For the subsequent biodegradation of such HSs, their oxidation is necessary, and under AD conditions, this type of conversion is less possible than in composting. In this regard, AWs are more attractive substrates for the formation of HSs via AD in comparison with lignocellulose raw materials. The accumulation of HSs during AD reduces the velocity and efficiency of the process due to the inhibition of the hydrolytic activity of cells participating in the functioning of methanogenic consortia [77][79]. A decrease in the HS concentrations in the digestate can also be observed with an increase in the temperature of the process due to the predominant formation of gas products (CH4 and CO2) and not HSs. In this regard, the HF of AW via AD proceeds at lower speeds than that via composting (Figure 2), but since AD is accompanied by the production of biogas, which can be used as biofuel, this process of HS acquisition retains interest.4. Hydrothermal Carbonation and Wet Torrefaction of AW
Hydrothermal carbonation (HTC), carried out in an aqueous environment at 170–280 °C and high pressure for several minutes or hours, can be used for the HF of AW at higher speeds than those in composting and AD (Table 2). Initially, a mass of AW is prepared, which is dried, crushed, and then mixed with water in different proportions [52][55][56][57][59]. HTC begins with the hydrolysis of high-molecular-weight compounds to monomers, and then the dehydration of monomers occurs, and the final product (hydrochar) containing HSs (Figure 1) is formed due to polymerization and aromatization processes [59]. Liquid (organic (acetic, propionic, and butanoic) acids [55], ketones, aromatic compounds, aldehydes, and alcohols) and gaseous (mainly CO2, CO, and CH4) products can accumulate in HTC. Depending on the conditions of the process and the AW used, it is possible to obtain final products with different yields, chemical compositions, and characteristics of the product with HSs [53]. Interestingly, the yield of hydrochar in the HTC of chicken manure (44.6%) was almost close to the result obtained with swine manure (43.4%) [56], while it exceeded the same characteristic of the HTC of lignocellulose raw materials by 4–5%. Among the HSs detected in the composition of hydrochar, 20% were compounds corresponding to HSs isolated from the soil, 30–40% were FLCs with a large molecular size, 10–25% of the compounds were characterized as “reduced quinones” with high aromaticity, and 12–23% were protein-like substances containing structures similar to aromatic amino acids (tyrosine and tryptophan) [58]. Wet torrefaction is a process similar to HTC [63][64] and can be conducted in aqueous or steam-water media at temperatures slightly lower than those for HTC (150–260 °C), with a processing time of up to 40 min (Table 2). The torrefaction temperature has a significant effect on the residual humidity, ash content, and yield of the resulting biochar produced from AW [63]. The yield of hydrochar with HSs decreases with an increasing process temperature [64]. The characteristics of the hydrochar should be carefully controlled. Despite incurring significant energy costs, as well as necessitating the use of special equipment that ensures the maintenance of the necessary temperature conditions of the process, HTC is currently attracting a lot of attention due to the high speeds of HF. The HS yields during the HF of various AWs via HTC (Table 2, Figure 2) are similar to those known for the natural HF of organic matter.5. HF of AW by Pyrolysis
Among the physical–chemical methods used for the HF of AW, pyrolysis is one of the most actively studied and consists of the heat treatment of dry raw materials in the absence of oxygen. Pyrolysis results in the formation of biochar with HSs; bio-oil with benzenes, alcohols, alkanes, alkenes, ketones, phenols, and poly aromatic hydrocarbons; and non-condensable gases (CO2, CO, CH4, H2, NH3, H2S, and hydrocarbons). The yield of biochar with HSs is determined by the conditions of the process and the composition of the AW used (Table 2). In the pyrolysis of AW, with an increase in temperature (from 400 to 700 °C) and the duration of the process (from 20 to 40 min), an increase in the proportion of the gas fraction among the products obtained (up to 40–60 wt.%) will manifest. The yield of biochar decreases with an increasing temperature (from 38 to 28 wt.%), and the degree of aromaticity of the HSs present in it increases [60]. Usually, up to 50% of the initial content of C, N, and S in the AW is lost in the form of volatile compounds, whereas the relative content of ash and metals increases by 2–2.4 times in comparison with AW [61][66]. The composition of HSs in biochar is similar to the composition of HSs in chernozem and peat, which are characterized by a large number of surface -COOH groups, providing them with a large capacity for cation exchange. The sum of all O-containing functional groups (C–O, C=O and COOH) in the composition of biochar HSs is lower than that of HSs from various types of soils, indicating the higher hydrophobicity of the biochar surface. In general, the HSs of biochar obtained as a product of the pyrolysis of AW are similar in their production rates and yields to the same parameters known for HTC (Figure 2); however, higher temperatures are required for pyrolysis. In this regard, pyrolysis, as a method for the HF of AW, refers to processes with high energy consumption but involving HSs, which, in their elemental composition and characteristics, are as close as possible to natural analogues coming from coal.6. Acid and Alkaline Hydrolysis as a Method for the HF of AW
As alternatives to high-temperature methods for the HF of AW, acid and alkaline hydrolytic processes are being actively investigated (Figure 1, Table 2). The efficiency of the conversion of AW into HSs using hydrolytic treatment depends on the type of processed mass [71]. The use of HCl or H2SO4 for the acid hydrolysis of AW is very effective for processing mass with a high content of polysaccharides [71]; however, other components of AW remain without effective hydrolytic action. The efficiency of the alkaline hydrolysis of AW depends on the alkaline agent used, for which KOH, NaOH, NH4OH, CaO, Ca(OH)2, and CaCO3 are applied in different concentrations (0.1–2 M) [24][72]. The temperature of the process (24–100 °C) and its duration (1–48 h) affect the extent of the HF of AW. These hydrolytic processes are interesting because they combine hydrolytic reactions, HF, and the accumulation of HSs in the liquid phase, which is usually carried out via the extraction of HSs from natural sources (coal and peat). According to the speed and the applied temperature conditions, hydrolytic processes occupy an intermediate position between biological (composting and AD) and physical–chemical high-temperature methods for the HF of AW (HTC and pyrolysis) (Figure 2). They ensure the immediate production of HSs dissolved in the reaction medium, without incurring significant energy and large time costs in the process. Innovative studies using subcritical water extraction, in which H2O and CO2 act like organic solvents such as methanol and chloroform, respectively, should be noted in this review [74]. A high yield of HSs from chicken manure (51 g/kg) was achieved during subcritical water extraction at 250 °C and a pressure of 50–60 atm. An increase in the temperature of the process (to 270 °C) led to a decrease in the content of HSs, just like what was noted earlier in other processes of HF. However, so far, such studies are rare, since the process is energy-intensive, requires subcritical extractants, and necessitates the use of expensive equipment that can generate high temperatures and pressure. Thus, alkaline hydrolysis is under active investigation because it enables the acquisition of HSs from AW quite easily, in a relatively short time, and at compromise temperatures. At the same time, the productivity of this process is lower than that of HTC and pyrolysis, and the issue of alkaline solid-phase waste disposal or application after separation of the liquid fraction with HSs remains unresolved.References
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68.
- Gerke, J. Concepts and misconceptions of humic substances as the stable part of soil organic matter: A review. Agronomy 2018, 8, 76.
- Wang, M.; Li, Y.; Peng, H.; Wang, J.; Li, Q.; Li, P.; Fan, J.; Liu, S.; Zheng, G. Review: Biotic and abiotic approaches to artificial humic acids production. Renew. Sustain. Energy Rev. 2023, 187, 113771.
- Chen, Y.; Inbar, Y.; Chefetz, B.; Hadar, Y. Composting and recycling of organic wastes. In Modern Agriculture and the Environment; Rosen, D., Tel-Or, E., Hadar, Y., Chen, Y., Eds.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1997; p. 71.
- Filho, J.F.D.C.L.; Thomason, W.E.; Evanylo, G.K.; Zhang, X.; Strickland, M.S.; Chim, B.K.; Diatta, A.A. The synergistic effects of humic substances and biofertilizers on plant development and microbial activity: A review. Int. J. Plant Soil Sci. 2020, 32, 56–75.
- Kulikova, N.A.; Perminova, I.V. Interactions between humic substances and microorganisms and their implications for nature-like bioremediation technologies. Molecules 2021, 26, 2706.
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239.
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621.
- Marcinčák, S.; Semjon, B.; Marcinčáková, D.; Reitznerová, A.; Mudroňová, D.; Vašková, J.; Nagy, J. Humic substances as a feed supplement and the benefits of produced chicken meat. Life 2023, 13, 927.
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Žatko, D.; Vaško, L. Therapeutic efficiency of humic acids in intoxications. Life 2023, 13, 971.
- Font-Palma, C. Methods for the treatment of cattle manure—A review. C J. Carbon Res. 2019, 5, 27.
- Bai, L.; Deng, Y.; Li, J.; Ji, M.; Ruan, W. Role of the proportion of cattle manure and biogas residue on the degradation of lignocellulose and humification during composting. Bioresour. Technol. 2020, 307, 122941.
- Jurgutis, L.J.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas production from chicken manure at different organic loading rates in a mesophilic full scale anaerobic digestion plant. Biomass Bioenergy 2020, 141, 105693.
- Santana, K.V.; Apolônio, F.C.; Wisniewski, A. Valorization of cattle manure by thermoconversion process in a rotary kiln reactor to produce environmentally friendly products. Bioenergy Res. 2020, 13, 605–617.
- Achinas, S.; Euverink, G.J.W. Rambling facets of manure-based biogas production in Europe: A briefing. Renew. Sustain. Energy Rev. 2020, 119, 109566.
- Scarlat, N.; Fahl, F.; Dallemand, J.F.; Monforti, F.; Motola, V. A spatial analysis of biogas potential from manure in Europe. Renew. Sustain. Energy Rev. 2018, 94, 915–930.
- Ali, M.Y.; Hassan, M.; Rahman, M.A.; Kafy, A.A.; Ara, I.; Javed, A.; Rahman, M.R. Life cycle energy and cost analysis of small scale biogas plant and solar PV system in rural areas of Bangladesh. Energy Procedia 2019, 160, 277–284.
- Prabakaran, R.; Valavan, S.E. Wealth from poultry waste: An overview. Worlds Poult. Sci. J. 2021, 77, 389–401.
- Manogaran, M.D.; Shamsuddin, R.; Yusoff, M.H.M.; Lay, M.; Siyal, A.A. A review on treatment processes of chicken manure. Clean. Circ. Bioecon. 2022, 2, 100013.
- Miroshnichenko, I.; Lomazov, V.; Petrosov, D.; Oskina, A. Bioenergetics’ potential of the poultry and swine wastes in Belgorod region of Russia. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012147.
- Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure by a leach-bed process coupled with side-stream membrane ammonia separation. Bioresour. Technol. 2018, 258, 41–47.
- Ramos-Suárez, J.L.; Ritter, A.; González, J.M.; Pérez, A.C. Biogas from animal manure: A sustainable energy opportunity in the Canary Islands. Renew Sustain. Energy Rev. 2019, 104, 137–150.
- Sawyerr, N.; Trois, C.; Workneh, T. Identification and characterization of potential feedstock for biogas production in South Africa. J. Ecol. Eng. 2019, 20, 103–116.
- Efremenko, E.; Senko, O.; Stepanov, N.; Aslanli, A.; Maslova, O.; Lyagin, I. Quorum sensing as a trigger that improves characteristics of microbial biocatalysts. Microorganisms 2023, 11, 1395.
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Eseva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794.
- Sheriff, I.; Yusoff, M.S.; Abd Manan, T.S.B.; Koroma, M. Microplastics in manure: Sources, analytical methods, toxicodynamic, and toxicokinetic endpoints in livestock and poultry. Environ. Adv. 2023, 12, 100372.
- Tawfk, A.; Eraky, M.; Osman, A.; Ai, P.; Zhou, Z.; Meng, F.; Rooney, D. Bioenergy production from chicken manure: A review. Environ. Chem. Lett. 2023, 21, 2707–2727.
- Zhu, N.; Gao, J.; Liang, D.; Zhu, Y.; Li, B.; Jin, H. Thermal pretreatment enhances the degradation and humification of lignocellulose by stimulating thermophilic bacteria during dairy manure composting. Bioresour. Technol. 2021, 319, 124149.
- Mei, J.; Ji, K.; Su, L.; Wu, M.; Zhou, X.; Duan, E. Effects of FeSO4 dosage on nitrogen loss and humification during the composting of cow dung and corn straw. Bioresour. Technol. 2021, 341, 125867.
- Zhang, Z.; Zhao, Y.; Wang, R.; Lu, Q.; Wu, J.; Zhang, D.; Nie, Z.; Wei, Z. Effect of the addition of exogenous precursors on humic substance formation during composting. Waste Manag. 2018, 79, 462–471.
- Xu, J.; Jiang, Z.; Li, M.; Li, Q. A compost-derived thermophilic microbial consortium enhances the humification process and alters the microbial diversity during composting. J. Environ. Manag. 2019, 243, 240–249.
- Niu, Q.; Meng, Q.; Yang, H.; Wang, Y.; Li, X.; Li, G.; Li, Q. Humification process and mechanisms investigated by Fenton-like reaction and laccase functional expression during composting. Bioresour. Technol. 2021, 341, 125906.
- Ren, X.; Wang, Q.; Zhang, Y.; Awasthi, M.K.; He, Y.; Li, R.; Zhang, Z. Improvement of humification and mechanism of nitrogen transformation during pig manure composting with Black Tourmaline. Bioresour. Technol. 2020, 307, 123236.
- Niu, Q.; Yan, H.; Meng, Q.; Wang, S.; Li, G.; Zhu, Q.; Li, X.; Li, Q. Hydrogen peroxide plus ascorbic acid enhanced organic matter deconstructions and composting performances via changing microbial communities. J. Environ. Manag. 2021, 295, 113126.
- Cui, P.; Liao, H.; Bai, Y.; Li, X.; Zhao, Q.; Chen, Z.; Yu, Z.; Yi, Z.; Zhou, S. Hyperthermophilic composting reduces nitrogen loss via inhibiting ammonifiers and enhancing nitrogenous humic substance formation. Sci. Total Environ. 2019, 692, 98–106.
- Cao, Y.; Wang, J.; Huang, H.; Sun, E.; Butterly, C.; Xu, Y.; He, H.; Zhang, J.; Chang, Z. Spectroscopic evidence for hyperthermophilic pretreatment intensifying humification during pig manure and rice straw composting. Bioresour. Technol. 2019, 294, 22131.
- Zhang, S.; Wei, Z.; Zhao, M.; Chen, X.; Wu, J.; Kang, K.; Wu, Y. Influence of malonic acid and manganese dioxide on humic substance formation and inhibition of CO2 release during composting. Bioresour. Technol. 2020, 318, 124075.
- Wang, L.; Zhao, Y.; Ge, J.; Zhu, L.; Wei, Z.; Wu, J.; Zhang, Z.; Pan, C. Effect of tricarboxylic acid cycle regulators on the formation of humic substance during composting: The performance in labile and refractory materials. Bioresour. Technol. 2019, 292, 121949.
- Wu, J.; Zhao, Y.; Yu, H.; Wei, D.; Yang, T.; Wei, Z.; Lu, Q.; Zhang, X. Effects of aeration rates on the structural changes in humic substance during co-composting of digestates and chicken manure. Sci. Total Environ. 2019, 658, 510–520.
- Li, C.; Li, H.; Yao, T.; Su, M.; Ran, F.; Li, J.; He, L.; Chen, X.; Zhang, C.; Qiu, H. Effects of swine manure composting by microbial inoculation: Heavy metal fractions, humic substances, and bacterial community metabolism. J. Hazard. Mater. 2021, 415, 125559.
- Jiang, Z.; Li, X.; Li, M.; Zhu, Q.; Li, G.; Ma, C.; Li, Q.; Meng, J.; Liu, Y.; Li, Q. Impacts of red mud on lignin depolymerization and humic substance formation mediated by laccase-producing bacterial community during composting. J. Hazard. Mater. 2021, 410, 124557.
- Yan, H.; Niu, Q.; Zhu, Q.; Wang, S.; Meng, Q.; Li, G.; Li, X.; Ma, C.; Li, Q. Biochar reinforced the populations of cbbL-containing autotrophic microbes and humic substance formation via sequestrating CO2 in composting process. J. Biotechnol. 2021, 333, 39–48.
- Wang, Y.; Zhang, C.; Zhao, Y.; Wei, Z.; Li, J.; Song, C.; Chen, X.; Zhao, M. Lignite drove phenol precursors to participate in the formation of humic acid during chicken manure composting. Sci. Total Environ. 2023, 874, 162609.
- Pan, C.; Zhao, Y.; Zhao, L.; Wu, J.; Zhang, X.; Xie, X.; Kang, K.; Jia, L. Modified montmorillonite and illite adjusted the preference of biotic and abiotic pathways of humus formation during chicken manure composting. Bioresour. Technol. 2021, 319, 124121.
- Jia, P.; Huang, Y.; Chen, M.; Qi, X.; Hou, H. Comprehensive evaluation of spent mushroom substrate-chicken manure co-composting by garden waste improvement: Physicochemical properties, humification process, and the spectral characteristics of dissolved organic matter. Environ. Sci. Pollut. Res. 2023, 30, 8987–8997.
- Hanc, A.; Enev, V.; Hrebeckova, T.; Klucakova, M.; Pekar, M. Characterization of humic acids in a continuous-feeding vermicomposting system with horse manure. Waste Manag. 2019, 99, 1–11.
- Wang, X.; Lyu, T.; Dong, R.; Liu, H.; Wu, S. Dynamic evolution of humic acids during anaerobic digestion: Exploring an effective auxiliary agent for heavy metal remediation. Bioresour. Technol. 2021, 320, 124331.
- Wang, X.; Muhmood, A.; Lyu, T.; Dong, R.; Liu, H.; Wu, S. Mechanisms of genuine humic acid evolution and its dynamic interaction with methane production in anaerobic digestion processes. Chem. Eng. J. 2021, 408, 127322.
- Rolando, C.; Elba, V.; Carlos, R. Anaerobic mono-digestion of Turkey manure: Efficient revaluation to obtain methane and soil conditioner. J. Water Resour. Prot. 2011, 3, 6987.
- Cestonaro, T.; de Mendonça Costa, M.S.S.; de Mendonça Costa, L.A.; Rozatti, M.A.T.; Pereira, D.C.; Lorin, H.E.F.; Carneiro, L.J. The anaerobic co-digestion of sheep bedding and 50% cattle manure increases biogas production and improves biofertilizer quality. Waste Manag. 2015, 46, 612–618.
- Renjiie, D.; Zijia, Z.; Sheng, L.; Yanfang, M.; Shan, L. Effects of hydrothermal pretreatments on the anaerobic digestion of pig manure and ecological safety of biogas slurry. TCSAE 2022, 38, 193–203.
- Lang, Q.; Zhang, B.; Liu, Z.; Jiao, W.; Xia, Y.; Chen, Z.; Li, D.; Ma, J.; Gai, C. Properties of hydrochars derived from swine manure by CaO assisted hydrothermal carbonization. J. Environ. Manag. 2019, 233, 440–446.
- Mau, V.; Arye, G.; Gross, A. Poultry litter hydrochar as an amendment for sandy soils. J. Environ. Manag. 2020, 271, 110959.
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85.
- Li, Q.; Zhang, S.; Gholizadeh, M.; Hu, X.; Yuan, X.; Sarkar, B.; Ok, Y.S. Co-hydrothermal carbonization of swine and chicken manure: Influence of cross-interaction on hydrochar and liquid characteristics. Sci. Total Environ. 2021, 786, 147381.
- Li, Q.; Lin, H.; Zhang, S.; Yuan, X.; Gholizadeh, M.; Wang, Y.; Xiang, J.; Hu, S.; Hu, X. Co-hydrothermal carbonization of swine manure and cellulose: Influence of mutual interaction of intermediates on properties of the products. Sci. Total Environ. 2021, 791, 148134.
- Lang, Q.; Guo, Y.; Zheng, Q.; Liu, Z.; Gai, C. Co-hydrothermal carbonization of lignocellulosic biomass and swine manure: Hydrochar properties and heavy metal transformation behavior. Bioresour. Technol. 2018, 266, 242–248.
- Song, C.; Shan, S.; Yang, C.; Zhang, C.; Zhou, X.; Ma, Q.; Yrjälä, K.; Zheng, H.; Cao, Y. The comparison of dissolved organic matter in hydrochars and biochars from pig manure. Sci. Total Environ. 2020, 720, 137423.
- Xiong, J.B.; Pan, Z.Q.; Xiao, X.F.; Huang, H.J.; Lai, F.Y.; Wang, J.X.; Chen, S.W. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water. J. Anal. Appl. Pyrolysis 2019, 144, 104692.
- Gómez, E.M.P.; Domínguez, R.E.; López, D.A.; Téllez, J.F.; Marino, M.D.; Almada, N.; Gange, J.M.; Moyano, E.L. Chicken litter: A waste or a source of chemicals? Fast pyrolysis and hydrothermal conversion as alternatives in the valorisation of poultry waste. J. Anal. Appl. Pyrolysis 2023, 169, 105796.
- Gascó, G.; Paz-Ferreiro, J.; Álvarez, M.L.; Saa, A.; Méndez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403.
- Zhou, S.; Liang, H.; Han, L.; Huang, G.; Yang, Z. The influence of manure feedstock, slow pyrolysis, and hydrothermal temperature on manure thermochemical and combustion properties. Waste Manag. 2019, 88, 85–95.
- Isemin, R.; Mikhalev, A.; Milovanov, O.; Nebyvaev, A. Some results of poultry litter processing into a fertilizer by the wet torrefaction method in a fluidized bed. Energies 2022, 5, 2414.
- Diker, İ.; Ozkan, G.M. An investigation on implementing wet torrefaction to dewatered poultry sludge. Biomass Conv. Bioref. 2022, 2022, 1–14.
- Arauzo, P.J.; Maziarka, P.A.; Olszewski, M.P.; Isemin, R.L.; Muratova, N.S.; Ronsse, F.; Kruse, A. Valorization of the poultry litter through wet torrefaction and different activation treatments. Sci. Total Environ. 2020, 732, 139288.
- Keskinen, R.; Hyväluoma, J.; Sohlo, L.; Help, H.; Rasa, K. Fertilizer and soil conditioner value of broiler manure biochars. Biochar 2019, 1, 259–270.
- Carneiro, J.S.D.S.; Lustosa Filho, J.F.; Nardis, B.O.; Ribeiro-Soares, J.; Zinn, Y.L.; Melo, L.C.A. Carbon stability of engineered biochar-based phosphate fertilizers. ACS Sustain. Chem. Eng. 2018, 6, 14203–14212.
- Atienza-Martínez, M.; Ábrego, J.; Gea, G.; Marías, F. Pyrolysis of dairy cattle manure: Evolution of char characteristics. J. Anal. Appl. Pyrolysis 2020, 145, 104724.
- Erdogdu, A.E.; Polat, R.; Ozbay, G. Pyrolysis of goat manure to produce bio-oil. Eng. Sci. Technol. Int. J. 2019, 22, 452–457.
- Zolfi Bavariani, M.; Ronaghi, A.; Ghasemi, R. Influence of pyrolysis temperatures on FTIR analysis, nutrient bioavailability, and agricultural use of poultry manure biochars. Commun. Soil Sci. Plant Anal. 2019, 50, 402–411.
- Bellera, C.; Abaalkheel, I.; Rovira, P.; Alrefai, A. Obtaining commercial humic products from uncomposted manures: Previous acid hydrolysis to enhance yields. Int. J. Recycl. Org. Waste Agric. 2015, 4, 219–231.
- Gayathri, B.; Srinivasamurthy, C.; Vasanthi, B.; Naveen, D.; Prakash, N.; Bhaskar, S. Extraction and charactrisation of humic acid from different organic wastes and its physico-chemical properties. Int. J. Chem. Stud. 2020, 8, 769–775.
- Mosa, A.; Taha, A.A.; Elsaeid, M. In-situ and ex-situ remediation of potentially toxic elements by humic acid extracted from different feedstocks: Experimental observations on a contaminated soil subjected to long-term irrigation with sewage effluents. Environ. Technol. Innov. 2021, 23, 101599.
- Sushkova, S.; Minkina, T.; Chaplygin, V.; Nevidomskaya, D.; Rajput, V.; Bauer, T.; Mazarji, M.; Bren, A.B.; Popov, I.; Mazanko, M. Subcritical water extraction of organic acids from chicken manure. J. Sci. Food Agric. 2021, 101, 1523–1529.
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453.
- Angelico, R.; Colombo, C.; Di Iorio, E.; Brtnický, M.; Fojt, J.; Conte, P. Humic substances: From supramolecular aggregation to fractal conformation—Is there time for a new paradigm? Appl. Sci. 2023, 13, 2236.
- Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Volikov, A.; Zhirkova, A.; Perminova, I. Strategies for variable regulation of methanogenesis efficiency and velocity. Appl. Microbiol. Biotechnol. 2022, 106, 6833–6845.
- Orlando, M.Q.; Borja, V.M. Pretreatment of animal manure biomass to improve biogas production: A Review. Energies 2020, 13, 3573.
- Wang, Z.; Li, X.; Siddiqui, M.A.; Liu, H.; Zhou, T.; Zheng, L.; Huang, S.; Gao, L.; Lin, C.S.K.; Wang, Q. Effect of humic substances on the anaerobic digestion of secondary sludge in wastewater treatment plants: A review. Environ. Chem. Lett. 2023, 21, 3023–3040.
