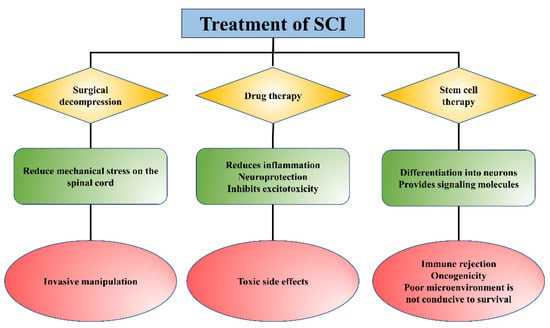
Spinal cord injury (SCI) is a severe neurological injury caused by traffic accidents, trauma, or falls, which leads to significant loss of sensory, motor, and autonomous functions and seriously affects the patient’s life quality. Although considerable progress has been made in mitigating secondary injury and promoting the regeneration/repair of SCI, the therapeutic effects need to be improved due to drug availability. Given their good biocompatibility, biodegradability, and low immunogenicity, injectable hydrogels can be used as delivery systems to achieve controlled release of drugs and other substances (cells and proteins, etc.), offering new hope for SCI repair.
- injectable hydrogel
- spinal cord injury
- delivery systems
1. Introduction
2. Types of Hydrogels
2.1. Natural Hydrogel
Natural hydrogels are formed by the polymerization of natural biomaterials or their derived derivatives, such as hyaluronic acid (HA), collagen, chitosan, agarose, alginate, etc. [27][52]. Natural polymers have inherent advantages, including abundant natural availability, specific molecules for cell adhesion, biodegradable, and biocompatibility [28][53], which can also reduce chronic inflammation or stimulation of the immune response due to their similarity to extracellular matrix (ECM) [29][54]. The various advantages of natural hydrogels make them widely used in the treatment of SCI. Kushchayev et al. found that HA hydrogel could protect the spinal cord from inflammation and reduce secondary injury in a SCI model of Sprague–Dawley (SD) rats [30][28], and injectable hyaluronate hydrogels with rapid self-healing ability served as a bridge and promoted angiogenesis, remyelination, and neural regeneration in SCI mice [31][29]. In addition, implantation of HA hydrogel in a rat model of dorsal hemisection injury of the spinal cord limited astrocyte activation and scar formation [32][30]. Collagen is highly abundant in the ECM and is an auspicious scaffold for promoting the repair, recovery, and regeneration of SCI [33][55]. Collagen hydrogel combined with small molecules, stem cells, or exosomes could promote neurogenesis, inhibit cell apoptosis and reduce glial scar production in SCI sites [34][35][36][31,32,33]. Chitosan has excellent biological properties, such as nontoxicity, biodegradation, and antibacterial activity [37][56]. Recently, chitosan-based hydrogel has attracted significant attention for SCI repair in nerve tissue engineering applications, which could inhibit neuroinflammation, promote the recovery of motor function, and prevent scar tissue formation in SCI animals [38][39][40][34,35,36]. In addition, Han et al. indicated that agarose scaffold containing Matrigel could support and enhance the regeneration of damaged spinal axons, and successfully reestablish the descending motor projection between motor cortical neurons and outside the lesion site [41][37].2.2. Synthetic Hydrogel
Synthetic hydrogels are formed through the physical or chemical cross-linking of synthetic polymers. Synthetic polymers are easier to mass-produce and have highly adjustable properties that can be customized to the needs of specific applications [42][60]. Polyethylene glycol (PEG), polyacrylamide (PAM), polyhydroxyethyl methacrylate (PHEMA), and poly-ε-caprolactone (PCL) are the common synthetic polymers [43][27]. PEG is a versatile polymer with no apparent toxicity or irritation [44][61], and hydrophilic PEG hydrogels can be fabricated through multiple crosslinking to produce scaffolds with different degradation rates and drug release rates [45][46][62,63]. Accumulating evidence has shown that PEG hydrogel could inhibit neuroinflammation in the early stages of SCI, repair membrane damage, promote axonal regeneration, and improve motor function after severe SCI [47][48][49][42,64,65]. Additionally, PAM hydrogels are widely used in the field of peripheral nerve regeneration because of their good biocompatibility, [50][68] but pure PAM hydrogels usually have poor mechanical properties and high brittleness [51][69]. Jiang et al. prepared PAM gels using DNA as a crosslinker, and when rat spinal cord cells were cultured in the hydrogel for a period of time, spinal cord neurons were observed to extend primary dendrites and shorter axons on the gel [52][43]. PHEMA has high mechanical strength and good biostability, which can be used as a basic ingredient in the treatment of SCI as a hydrogel.2.3. Composite Hydrogel
Composite hydrogels combine the biocompatibility of natural hydrogels with the tunable mechanical and physical properties of synthetic hydrogels, thus holding promise for a wide range of applications in SCI [43][27]. Zhao et al. developed a double crosslinked biomimetic composite hydrogel comprised of acellularized spinal cord matrix, gelatin-acrylated-β-cyclodextrin-polyethene glycol diacrylate, and WAY-316606, which could recruit endogenous neural stem cells, improve neuronal differentiation, and induce neural tissue regeneration and functional recovery after SCI with no obvious cytotoxicity [53][49]. In addition, a novel multifunctional composite hydrogel consisting of fibrin hydrogels and functionalized self-assembling peptides promoted spinal cord regeneration by guiding regenerative tissue, accelerating axonal regeneration and remyelination, and promoting angiogenesis [54][50].3. Application of Hydrogel as a Delivery System in SCI
Injectable hydrogels as delivery systems can encapsulate different substances, such as cells, drugs, and biomolecules (Figure 2) and release them slowly and controllably at the site of injury to improve drug utilization and efficacy (Table 12). Thus, hydrogels have received accumulating attention and made many breakthroughs in the treatment of SCI as a drug delivery system.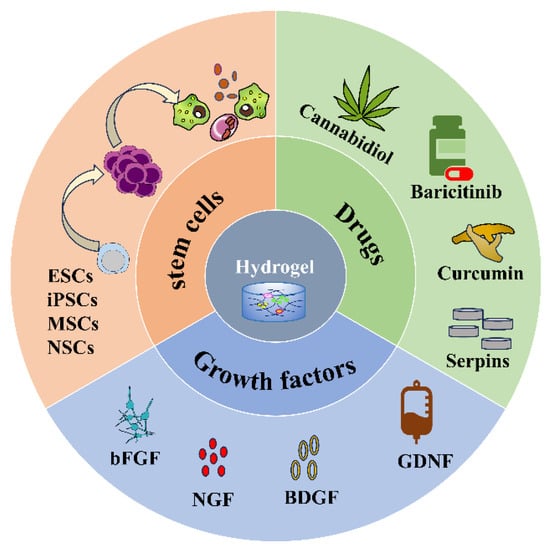
| Encapsulated Substances | Hydrogel Composition | Specific Substances | Function | Ref |
|---|---|---|---|---|
| Stem cells | PLGA; TA; oxidized dextran (Dex) and hyaluronic acid-hydrazide | NSCs | Promote the differentiation of NSCs into neurons while inhibiting the differentiation of astrocytes | [55][72] |
| HA; collagen | NSCs | Promote NSCs to differentiate into neuron-like cells and play neuroprotective roles | [56][73] | |
| Gelatin; peroxidase (HRP) and galactose oxidase (GalOx) | MSCs | Enhance the neural differentiation and functional recovery of SCI; | [57][74] | |
| ECM | Human iPSCs | Reduces inflammation, enhance nerve regeneration, and significantly improve movement | [58][75] | |
| Gelatin; methacrylic | ADSCs | Promote axon growth, reduce neuroinflammation, and ultimately improve motor function in SCI rats | [59][76] | |
| Drugs | Alginate; chitosan | Erythropoietin (EPO) | Improve tissue repair and histopathological appearance of the spinal cord at the site of injury | [60][77] |
| Chitosan; collagen | Serpins | Improve the neurological and motor function and reduce tissue damage caused by in-flammation in SCI rats | [61][78] | |
| Chitosan | Cannabidiol (CBD) | Reduce apoptosis, improve neurogenesis by enhancing mitochondrial biogenesis | [62][79] | |
| PLGA; PEG | Baricitinib | Reduce neuronal apoptosis and promote functional recovery in SCI rats | [63][80] | |
| Chitosan | Curcumin | Favor functional recovery of SCI rats | [64][81] | |
| Growth factors | Heparin-poloxamer | bFGF and NGF | Improve neuronal survival, inhibit reactive astrogliosis, and promote recovery of motor performance | [65][82] |
| Alginate | GDNF | Stimulate neurite growth and functional recovery | [66][83] | |
| Naphthalene acetic acid-phenylalanine-phenylalanine-glycine | Platelet-derived growth factor (PDGF) | Inhibit M1 macrophage infiltration and extrinsic or intrinsic cells apoptosis | [67][84] | |
| Heparin-Laponite | FGF4 | Inhibit inflammatory response, increase myelination regeneration, and reduce glial/fibrotic scarring | [68][85] |
3.1. Stem Cells
3.2. Drugs
Though many drugs have been tested in experimental models of SCI, the clinical translation of established therapeutic agents for the treatment of SCI remains challenging. First, most systemic drugs cannot reach the site of injury due to the impermeability of the blood–spinal cord barrier [73][90]. In addition, most drugs usually have short half-lives and require high doses and/or frequent administration to reach therapeutic concentrations at the site of injury, which can lead to harmful side effects and may lead to sustained inflammatory activation [74][91]. Injectable hydrogels with sustained drug delivery properties, degradability, and tunable physical properties can overcome and optimize these shortcomings either as a model of transport for the drug itself or as a carrier for drug-loaded particles/carriers [22][75][22,92]. Erythropoietin (EPO) is a growth factor that exhibits neuroprotective effects in the treatment of SCI [76][93]. Studies have shown that EPO-chitosan/alginate (EPO-CH/AL) hydrogels have controlled release characteristics for EPO, and EPO-CH/AL hydrogels significantly improve tissue repair and the histopathological appearance of the spinal cord at the site of injury [60][77]. Serine protease inhibitors (serpins) are “suicide” inhibitors with a highly conserved structure, which prevents excessive bleeding or clotting.3.3. Growth Factors
Growth factors (GFs) could stimulate the growth of specific tissues, direct specific cellular responses in the microenvironment, and promote axonal regeneration [77][96]. Commonly used GFs include fibroblast growth factor (bFGF), nerve growth factor (NGF), BDNF, and glial neurotrophic factor (GDNF), etc., all of which are associated with neurodevelopment and neurogenesis [78][97]. The use of GFs for SCI has been shown to promote axonal regeneration and functional recovery [79][98]. However, direct administration of GFs is limited by their rapid degradation and dilution at the site of injury [65][82]. As a biocompatible biological scaffold, hydrogels have a high affinity for GFs and can stably control the release of GFs, avoiding the side effects of high GFs concentrations at the injection site and protecting them from enzymatic hydrolysis [80][99]. Thus, controlled delivery of multiple GFs to the lesion is becoming an attractive strategy for repairing SCI. For instance, Hu et al. developed a heparin-poloxamer (HP)-based hydrogel for the delivery of bFGF and NGF, which significantly improved neuronal survival, inhibited reactive astrogliosis, and promoted recovery of motor performance in SCI rats [65][82]. Ansorena et al. found that GDNF-loaded injectable alginate hydrogels stimulated neurite growth and functional recovery after SCI with more growing neuritis at the lesion site [66][83].4. Therapeutic Mechanism of Injectable Hydrogels in SCI
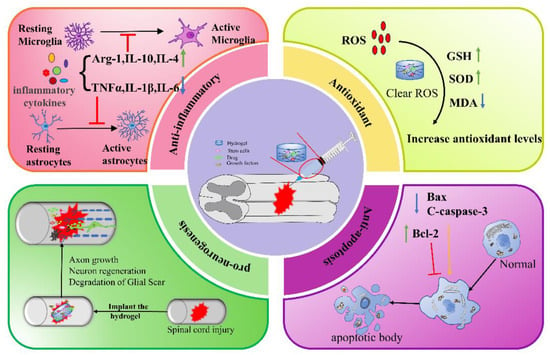
4.1. Anti-Inflammation
The inflammatory response following SCI is a complex process coordinated by many cell types and inflammatory factors, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon-γ (IFN-γ), etc. [81][122]. TNF-α and IL-6 are significantly upregulated around the area of SCI from 3 to 24 h [5]. Although inflammation is a universal consequence of systemic trauma and an essential defense mechanism for the host, ref. [82][123] inflammation in SCI is a double-edged sword [83][124]. For one thing, the inflammatory response is necessary to effectively remove tissue debris and promote wound healing and tissue repair. For another, various factors harmful to neurons, glial cells, axons, and myelin are also released during the inflammatory response. With the increase of inflammatory cytokines, the toxic microenvironment leads to the formation of cavities and glial scars, thus inhibiting the recovery of nerve function [84][125]. Microglia/macrophage-mediated neuroinflammation persists for a long period of time and affects SCI repair. Therefore, suppression of chronic inflammation is favorable for the recovery of SCI, but the timing of inflammatory interventions should be kept in mind [85][126]. Hydrogel can control the release of stem cells, anti-inflammatory drugs, GFs, etc., and improving the local microenvironment in the lesion, which has broad application prospects in SCI treatment [86][127].4.2. Antioxidant
SCI is accompanied by the loss of ionic homeostasis, glutamate excitotoxicity, mitochondrial dysfunction, and oxidative stress [71][88]. The accumulation of large amounts of reactive oxygen species (ROS) leads to massive neuronal death, which further leads to secondary damage in SCI. Inhibition of post-injury peroxidation of biomolecules through effective antioxidant interventions will be a strategy for the treatment of SCI [87][128]. Therefore, functionalized hydrogels with free radical scavenging capacity or loaded with antioxidants will be beneficial for SCI recovery and functional reconstitution [88][129]. For example, the manganese-dioxide-nanoparticle-dotted (MnO2NPs) HA hydrogel prepared by Li et al. regulated the ROS microenvironment of SCI, thereby effectively improving the viability of MSCs and synergistic promotion of spinal cord repair [89][108]. Liu et al. prepared a N-acryloylglycinamide/methacrylic gelatin/laponite/tannic acid (TA) hydrogel combined with MSC-derived small extracellular vesicles (MSC-sEVs), which can realize local, sustainable, and stable delivery of MSC-sEVs at the SCI site, effectively scavenge free radicals, and reduce the expression of 4-hydroxynonenal and 8-hydroxydeoxyguanosine caused by oxidative stress [90][109].4.3. Anti-Apoptosis
Apoptosis is a physiological process that occurs in cell development, but damaged cells die during apoptosis [91][130]. There are two pathways of cell death in the injured spinal cord: immediate necrosis and delayed apoptosis of cells. The latter lasts for approximately 14 days after trauma and involves neurons and glial cells that are far from the traumatic area [92][131]. Apoptosis may lead to neuronal cell death and play an important role in the pathogenesis of neurological disorders [93][132]. The main genes involved in apoptosis are Bcl-2 (apoptosis inhibitor) and Bax (apoptosis promoter). A growing number of studies have shown that injectable hydrogels can reduce neuronal apoptosis and promote neuronal cell survival, which has great potential in SCI treatment [94][133]. For example, Yuan et al. developed a CaNeu hydrogel as a delivery vehicle for ADSCs, and studies have shown that this hydrogel significantly inhibited neuroinflammation and cell apoptosis by reducing the expression of the pro-apoptotic protein Bax at the lesion site, while increasing the expression level of anti-apoptotic protein Bcl-6 [59][76]. Li et al. co-immobilized umbilical cord MSCs and bFGF in ECM and HP to form a bioactive, heat-sensitive hydrogel, which exerted promising utility for the functional recovery of SCI by reducing cell apoptosis and improving mitochondrial function [95][114].4.4. Pro-Neurogenesis
Severe and chronic SCI are often associated with the permanent loss of neurological function, mainly due to the failure of injured axons to regenerate and rebuild functional connections and the loss of neurons. Therefore, promoting neural regeneration is a feasible idea for improving sensorimotor recovery of SCI [2]. Neural regeneration is the regeneration and repair of damaged neural tissue (neurons, axons, synapses, and glial cells) after injury [96][134], which includes the elongation of axons, the germination and growth of new axons, or the regeneration of neuronal cells [97][135]. Thus, regeneration, including both neuronal and axonal regeneration, is a complex biological process that requires joint coordination [98][136]. Current drug- or cell-based SCI therapies fail to provide topographic guidance for regenerating neurons and result in random growth and poor therapeutic efficacy [96][134]. Injectable hydrogels as biological scaffolds can not only load drugs or cells but can also create structures that allow neuronal growth and guide axon regeneration throughout the injury site [99][137]. Axons are the tiny nerve fibers that connect neurons and allow them to communicate [100][138]. Zhang et al. found that GelMA hydrogel lengthened the axons of mouse neurons, increased the expression of growth-related protein GAP43, and promoted the recovery of neurological function of SCI mice [101][118]. Fan et al. demonstrated that gelatin methacrylate (GM)-modified hydrogels immobilized BMSC exosomes and promoted axon outgrowth and neural synaptic network formation in vitro.5. Combination Therapy
Currently, low-frequency pulsed electromagnetic field (LFPEMF) is a clinically used non-invasive therapeutic measure for neural repair that has been shown to prevent inflammation and oxidative stress, and it exhibits powerful neuroprotective effects in the nervous system [102][139]. Conductive hydrogels are attractive candidates for accelerating SCI repair because they match the electrical and mechanical properties of the neural tissues [103][140]. Therefore, hydrogels combined with electromagnetic stimulation (ES) to treat SCI have become an interesting strategy. For example, Liu et al. demonstrated that combined with ES by electrode needles, thermosensitive-electroactive-hydrogel-loaded NGF significantly inhibited astrocyte differentiation and restored spinal circuitry and locomotor function by stimulating endogenous neurogenesis in a rat SCI model [104][141]. Moreover, implantation of an IONP-embedded gelatin–genipin hydrogel system along with MF (17.96 μT, 50 Hz uniform EMF) exposure modulated the microenvironment, making it conducive to neural repair and regeneration after SCI in rats [105][142].6. Conclusions
In summary, natural, synthetic, and composite injectable hydrogels can all be used as delivery systems to encapsulate stem cells, drugs, or GFs for a wide range of applications in SCI therapy. The mechanisms by which hydrogels promote SCI repair include anti-inflammation, anti-oxidation, anti-apoptosis, and pro-neurogenesis (Figure 3), etc. In addition, hydrogel combined with electromagnetic stimulation or phototherapy can also improve the repair of SCI. Although much progress has been made in the study of injectable hydrogels for SCI, there are still certain limitations.