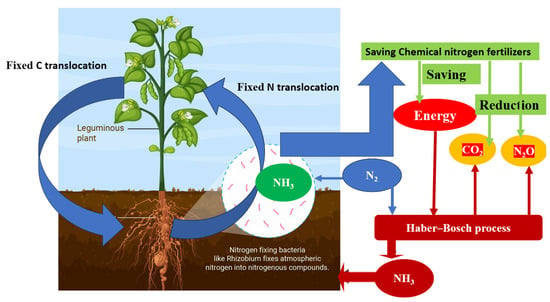
Nitrogen fixation has the potential to address the global protein shortage by increasing nitrogen supply in agriculture. However, the excessive use of synthetic fertilizers has led to environmental consequences and high energy consumption. To promote sustainable agriculture, alternative approaches such as biofertilizers that utilize biological nitrogen fixation have been introduced to minimize ecological impact. Understanding the process of biological nitrogen fixation, where certain bacteria convert atmospheric nitrogen into ammonia, is crucial for sustainable agriculture. This knowledge helps reduce reliance on synthetic fertilizers and maintain soil fertility. The symbiotic relationship between Rhizobium bacteria and leguminous plants plays a vital role in sustainable agriculture by facilitating access to atmospheric nitrogen, improving soil fertility, and reducing the need for chemical fertilizers.
- nitrogen fertilizer
- Rhizobium
- legume
1. Introduction
Nitrogen, also known as N2, is a vital element that makes up 78% of Earth’s atmosphere [8]. It plays a crucial role in plant growth, with plants requiring larger amounts of nitrogen compared to other elements [9,10]. However, plants cannot directly use nitrogen gas due to its stability and strong triple bond between nitrogen atoms. They need nitrogen to be converted into reduced forms, which they obtain from various sources such as ammonia or nitrate fertilizers, organic matter decomposition, natural processes like lightning, and biological nitrogen fixation [11]. The production of fertilizers, insecticides, irrigation, and machinery for the green revolution heavily relies on fossil fuels, with approximately 80% of the world’s fossil energy being used [12,13]. Over the past four decades, global nitrogen fertilizer usage has significantly increased, contributing to over half of the energy consumed in agriculture [14,15]. The manufacturing process for nitrogen fertilizer using the Haber–Bosch process alone emits approximately 465 teragrams of carbon dioxide annually, making it a significant source of greenhouse gas emissions [16,17,18]. The nitrogen fertilizer industry has been found to contribute up to 1.2% of total greenhouse emissions resulting from human activities [12,19,20,21].
The fertilizer industry heavily relies on energy-intensive technologies for agricultural production, including the manufacture of nitrogen fertilizers and pesticides. Global nitrogen fertilizer consumption reached approximately 108 million tons in 2019 and slightly increased to 110 million tons in 2020–2021, with a projected annual growth rate of 4.1% until 2025–2026 [23]. However, the scarcity of fossil energy is a significant challenge that the world may face [24,25]. The excessive use of nitrogen fertilizer can have significant environmental consequences. One major issue is nutrient runoff, where high levels of nitrogen and phosphorus from synthetic fertilizers can be washed into nearby water bodies, causing eutrophication, harmful algal blooms, oxygen depletion, and disruption in aquatic ecosystems [26,27,28]. Another problem is soil degradation. Synthetic fertilizers primarily focus on macronutrients like nitrogen, phosphorus, and potassium, neglecting other essential micronutrients. This imbalanced nutrient application can deplete soil organic matter, damage its physical structure, decrease beneficial microbial activity, and reduce overall fertility over time [29,30,31]. Biodiversity loss is also a concern. Nutrient runoff leading to eutrophication can harm aquatic life, resulting in a decline in fish populations and other species. Moreover, the loss of soil fertility due to synthetic fertilizers can negatively affect soil organisms crucial for maintaining healthy soil and biodiversity, such as earthworms, beneficial insects, and microorganisms [32]. The production and distribution of synthetic fertilizers contribute to greenhouse gas emissions and air pollution. This energy-intensive process relies on fossil fuels and releases carbon dioxide (CO2), nitrogen oxides (NOx), and methane (CH4), exacerbating climate change [33].
To mitigate the consequences of unsustainable agricultural practices, several sustainable alternatives have been developed. These include organic farming, crop rotation, cover cropping, and the use of natural fertilizers such as compost and manure. By reducing reliance on synthetic fertilizers, these practices promote environmental sustainability. Additionally, alternatives like biofertilizers and biopesticides have been embraced in modern agriculture. These options help alleviate energy consumption, greenhouse gas emissions, and negative impacts of excessive nitrogen waste in agroecosystems [35,36]. Biofertilizers and biopesticides encourage biological nitrogen fixation, a process facilitated by microorganisms that significantly contribute to the nitrogen cycle and overall nitrogen balance. Global terrestrial biological nitrogen fixation is estimated to range from 52 to 130 teragrams (Tg) of nitrogen per year [37,38,39]. Biological nitrogen fixation aligns with the principles of green engineering as it relies on renewable sunlight and has minimal ecological impact [40,41].
2. Biological Nitrogen Fixation Systems
Rhizobium–Legume Symbiotic Relationship and Environmental Stress
The Rhizobium–legume symbiotic relationship is a crucial association between leguminous plants and nitrogen-fixing bacteria called rhizobia. Rhizobia play a vital role in nitrogen fixation and sustainable agriculture [49]. They colonize legume roots, forming nodules, where they convert atmospheric nitrogen into a plant-usable form through nitrogen fixation [50]. In return, legumes provide energy to the rhizobia through photosynthesis. Nitrogen fixation is significant for sustainable agriculture as it enhances soil fertility by converting inaccessible atmospheric nitrogen into a usable form. This reduces reliance on synthetic fertilizers, which have adverse environmental impacts [51]. The symbiosis between rhizobia and legumes also promotes leguminous crop growth and development, leading to improved crop yield. Moreover, this relationship contributes to sustainable agriculture by reducing the need for chemical fertilizers, mitigating soil degradation, and enhancing overall soil health. Legume plants can serve as valuable cover crops or be integrated into crop rotations, thus enhancing the sustainability and productivity of agricultural systems [52,53,54]. Several environmental conditions can negatively affect symbiotic nitrogen fixation in legumes, such as nitrogen availability, soil acidity, salinity, and low soil temperature [55,56,57]. These factors can impact various aspects, including rhizobial survival in the soil, the infection process, nodule development, nodule function, and indirect effects on host plant growth [58,59,60]. Nitrogen availability plays a crucial role in legume–rhizobia symbiosis, as higher doses of nitrogen fertilizer can hinder successful symbiotic establishment [60]. When the soil has a high nitrogen content, especially during the period between seed inoculation and germination, it presents challenges for establishing functional symbiosis [61,62]. Excessive nitrogen in the soil can reduce the reliance of plants on nitrogen fixation and limit root nodule development. Soil acidity and salinity can also impede symbiotic nitrogen fixation by creating unfavorable conditions for rhizobial survival and root infection. This hampers nodule development and results in fewer functional nodules [63].3. Effects of N Fertilizer on Rhizobium–Legume Molecular Signaling
In the symbiotic relationship between legumes and rhizobial bacteria, several molecular signals are involved in the recognition and initiation processes. These signals facilitate the establishment of a beneficial relationship between legume plants and rhizobial bacteria by guiding their interactions and ensuring successful symbiosis (Figure 13). The key players in this process are as follows: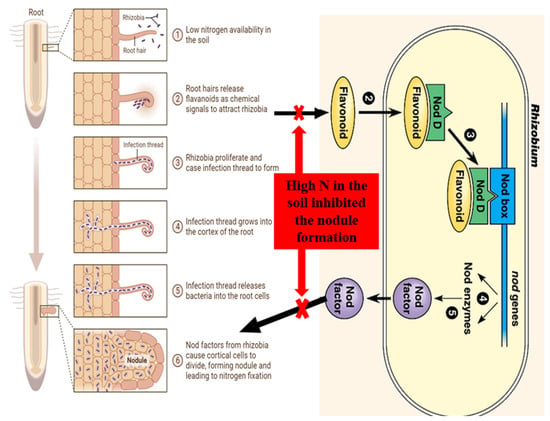
3.1. Isoflavonoids
3.2. Nod Factors
Nod factors are lipochitooligosaccharides produced by rhizobia, bacteria that respond to (iso)flavonoids by secreting nod factors as signaling molecules. Nod factors induce various responses in legume plants, including root hair deformation, curling, and the initiation of nodule formation, as shown in Figure 13 [88,89,90]. (Iso)flavonoids act as chemoattractants, guiding rhizobia towards the roots [91]. Bacterial chemoreceptors perceive (iso)flavonoids, initiating chemotaxis towards the chemoattractant source [92]. The specificity of the symbiosis lies in the ability of (iso)flavonoids to activate specific nod factor receptors on root hairs. The binding of nod factors to these receptors triggers a signal transduction pathway. The interaction between root hairs and rhizobia leads to the formation of infection threads, specialized structures that allow bacteria to penetrate root tissues [93,94]. Infection threads provide a protected pathway for rhizobia to move towards the inner regions of the root [95,96]. Within the root, rhizobia colonize nodule primordia and induce their differentiation into mature nodules. Infection threads guide rhizobia towards the nodule primordia, where they undergo morphological changes [97]. The nodule meristem, formed through host plant cell division, provides a continuous source of new cells for nodule formation and growth. Rhizobia within nodules differentiate into bacteroids, highly specialized forms enclosed within symbiosomes derived from host plant cells [98]. Symbiosomes facilitate nutrient exchange. Nitrogen fixation occurs within nodules, converting atmospheric nitrogen into a usable form. The plant supplies carbon sources and essential nutrients to rhizobia. High levels of nitrogen fertilization can negatively impact nod factor production, leading to decreased nodule formation and effectiveness [99]. Excessive nitrogen availability suppresses nod factor synthesis genes [100]. This reduction in nod factor production affects symbiosis and overall nitrogen fixation [101]. The impact of nitrogen fertilizers varies based on factors such as fertilizer type, concentration, soil conditions, rhizobial strain, and the leguminous plants involved. Timing of fertilizer application is crucial, with excessive nitrogen during early stages having more detrimental effects [102].3.3. Nodulation Receptor Kinases (NORKs)
Nodulation Receptor Kinases (NORKs) are a group of receptor proteins found in the root hairs of leguminous plants [104]. They play a crucial role in the symbiotic relationship between legumes and nitrogen-fixing bacteria, known as rhizobia [105]. NORKs are responsible for recognizing and binding to specific signaling molecules called nod factors, which are produced by rhizobia. This recognition and binding event initiate a series of downstream signaling events that trigger physiological and developmental responses in both legume plants and rhizobia [106]. The signaling cascade initiated by NORKs leads to nodule formation on the roots of leguminous plants [107]. NORKs belong to a larger family of receptor proteins called Receptor-Like Kinases (RLKs). RLKs are transmembrane proteins with a receptor domain on the extracellular side of the cell membrane and a kinase domain on the intracellular side. This dual nature allows RLKs to perceive external signals, such as nod factors, and transmit them to the cell by phosphorylating downstream proteins [108]. The precise mechanisms by which NORKs transmit these signals are still being investigated, but they are believed to interact with other proteins and enzymes to relay nod factor signals into the cell [109].3.4. Calcium Spikes Play a Crucial Role in Symbiotic Signaling
Calcium spikes play a crucial role in symbiotic signaling and the modulation of gene expression in interactions between legumes and rhizobia [116]. These spikes are triggered by the recognition of nod factors by nodulation receptor kinases (NORKs) in the root hairs, leading to oscillations in calcium levels [117]. The frequency and pattern of these spikes are vital for accurate signaling and changes in gene expression during symbiosis [117]. Calcium spikes act as second messengers in the signaling pathway, transmitting the perception of nod factors from the membrane to the nucleus [118]. They function as a “calcium clock”, regulating the timing and duration of subsequent events [119]. The precise mechanism behind calcium spiking is currently under investigation, but it is known that NORKs play a crucial role in initiating the oscillations [120]. When nod factors bind to NORKs, specific channels or pumps in the plasma membrane are activated, resulting in the influx or release of calcium ions into the cytosol. This leads to temporary increases in calcium levels, known as calcium spikes. These spikes are associated with the activation of symbiotic genes, promoting the expression of nodulation-related proteins and transcription factors [121].3.5. Cytokinins and Auxins
Cytokinins and auxins are vital plant hormones involved in the nodulation process [130,131,132,133,134]. Cytokinins stimulate cell division and growth, while auxins drive swelling and deformation, leading to the formation of nodules [135]. During nodulation, leguminous plants recognize nod factors, which trigger the production of cytokinin in the root hairs and cortical cells. Increased cytokinin levels promote cell division in the root cortex, forming a nodule primordium. Cytokinins also assist in the swelling and deformation of root hairs, facilitating rhizobial infection [134,136]. On the other hand, auxins play a crucial role in nodulation by promoting cell elongation and differentiation [131]. Nod factor signaling triggers the synthesis and redistribution of auxins in the root hairs and cortical cells [131]. Auxins contribute to the deformation of root hairs, aiding rhizobial infection [137]. They also promote the growth of nodule primordia by stimulating cell elongation and division in the proliferating zone [130]. The interaction between cytokinins and auxins is vital for nodule formation, as they work together to regulate cell division, elongation, and differentiation, ensuring proper nodule growth [138].3.6. Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) play a vital role in the symbiotic relationship between legumes and rhizobia [147,148,149,150]. ROS are produced when legumes detect rhizobia through nod factors, initiating a signaling cascade [151]. These ROS molecules act as signaling molecules themselves, coordinating a complex dialogue that promotes the symbiotic relationship [152]. Receptor proteins on legume root cells recognize nod factors and activate enzymes involved in ROS production [153]. ROS, including hydrogen peroxide (H2O2), superoxide (O2−), and hydroxyl radicals (OH•), have both positive and negative effects within cells. In the legume–rhizobia symbiosis, ROS production is crucial for recognition, cell wall modifications, and infection thread formation [154,155,156]. ROS also regulate gene expression, defense responses, and root nodule formation [157,158,159]. While ROS are important, excessive production can cause oxidative damage to both the legume host and rhizobia. Therefore, the ROS signaling pathway is tightly regulated [160]. Understanding the role of ROS in legume–rhizobia symbiosis provides insights into the molecular mechanisms and potential for enhancing crop productivity through improved nitrogen fixation. Nitrogen fertilizer can influence ROS levels in plants. E4. Effects of N Fertilizer on Rhizobial Motility
Motility in rhizobia refers to their ability to actively move in response to external stimuli, usually by using flagella. Rhizobia are bacteria that form mutually beneficial relationships with legume plants, helping with nitrogen fixation [163,164,165]. However, excessive nitrogen fertilization can have negative effects on rhizobial populations, motility, and their symbiotic relationship with legume plants. Studies have shown that high nitrogen levels can reduce the density, motility, and diversity of free-living rhizobia, which can harm soil health and plant productivity [166]. The effects of excessive nitrogen fertilization include population dynamics, reduced motility, inhibition of nodulation, altered mutualistic relationships, and environmental concerns [163]. Excess nitrogen can change soil pH, inhibiting rhizobial growth [167,168]. Additionally, high nitrate levels can provide an alternative nitrogen source for plants, reducing their reliance on rhizobial symbiosis and further impacting Rhizobium populations [169,170]. High ammonium concentrations from excessive nitrogen fertilization can decrease rhizobial motility, impairing their ability to effectively colonize plant roots [171]. Managing nitrogen fertilizer application is crucial to ensure optimal nodulation and symbiotic nitrogen fixation in legume crops while minimizing negative effects on rhizobia and the environment [172,173]. The contribution of motility to symbiotic recognition is essential in establishing and maintaining beneficial relationships between organisms [174,175,176].5. Effect of N Fertilizer on Root-Hair Curling, Infection Thread Formation and Nodulation
Extensive research has been conducted on the impact of nitrogen fertilizer on various aspects of legume growth, including root-hair curling, infection thread formation, and nodulation. Leguminous plants rely on symbiotic nitrogen fixation with rhizobia to meet their nitrogen requirements. However, excessive use of nitrogen fertilizer can disrupt this symbiotic association [59,177,178,179,180]. The effect of nitrogen fertilizer on root-hair curling and rhizobial infection varies depending on factors such as plant species, soil conditions, and the timing, form, and amount of nitrogen application [59,181]. Abdel Wahab et al. [59] reported that nitrogen fertilizers can hinder multiple stages of legume nodulation, including root-hair infection. Root hairs play a crucial role in the interaction between legume roots and rhizobia [182]. High concentrations of nitrogen fertilizers, particularly nitrate, can significantly reduce root elongation and curling, negatively impacting the ability of rhizobia to colonize and infect the roots [183,184]. The addition of nitrate or urea has been found to have significant effects on root-hair curling, infection thread formation, and nodulation in various plant species. Nitrogen fertilizers can directly impact nodule development, leading to the abortion of infection. Additionally, their presence can reduce the proliferation and multiplication of free-living rhizobia in the soil, delaying or inhibiting nodule formation [186,187,188,189]. Nitrogen fertilizer application also leads to premature nodule senescence and feedback inhibition of nitrogenase activity [190,191,192,193,194,195,196]. The inhibitory effects of nitrogen fertilizers on nodulation are likely plant-mediated, and different strains of rhizobia exhibit varying degrees of tolerance to these effects [197,198]. Furthermore, the form of nitrogen also plays a role in its suppressive effects on nodulation, with nitrate being more inhibitory compared to ammonia or urea [195].6. Effect of Nitrogen Fertilizers on Nodule Physiology
6.1. Nodule Nitrate Reductase
Early studies revealed that the addition of nitrate or ammonium to soybean plants resulted in a decrease in nitrate reductase activity in nodules [209]. Further research investigated the effects of nitrate on symbiotic properties using nitrate-reductase-deficient mutants of cowpea rhizobia and Rhizobium trifolii [210]. The study found that nitrate inhibited initial nodulation. It has been found that nitrate inhibits nitrogen fixation in cowpea and lupine nodules, irrespective of the presence or absence of nitrate reductase activity [211]. A significant reduction in nodule weight was observed in soybean plants exposed to high concentrations of nitrate, as reported by Streeter [212]. It is worth noting that the inclusion of sucrose has been found to enhance nitrogenase activity and decrease nitrite accumulation [213].6.2. Leghemoglobin
The first plant hemoglobin was discovered in soybean nodules [226]. It has been demonstrated that this hemoglobin forms a reversible compound with molecular oxygen [227]. Hemoglobin in Pisum sativum nodules was also discovered, and its content was found to be related to nitrogen fixation. This hemoglobin was named ‘’leghemoglobin’’ for legume nodules by Virtanen and Laine [228]. However, the role of this protein in facilitating oxygen diffusion to the bacteroids has been firmly established [229,230]. Hemoglobin was also discovered in the nodules of Parasponia andersonii, a nonlegume plant belonging to the Cannabaceae family [231]. These nodules form a symbiotic relationship with bradyrhizobia. Hemoglobin was also found in the nodules of actinorhizal plants such as Casuarina glauca, Myrica gale, and Alnus glutinosa [232].6.3. Nitrogenase Activity
Studies have been conducted over several decades to understand the inhibition of nitrogen fixation by mineral nitrogen. This is of increasing importance as environmentalists and agricultural scientists seek ways to reduce fertilizer use in field crops. If we can manipulate symbiosis to overcome this inhibition, legumes could increase the amount of nitrogen derived from nitrogen fixation, which would have a greater impact on soil nitrogen levels. Several hypotheses have been proposed to explain the mechanism of nitrate inhibition of nitrogenase activity in legumes. These include changes in plant carbohydrate distribution, resulting in energy and carbon deficiencies in nodules [203], inhibition of nitrogenase or leghemoglobin synthesis [243,244], inhibition of nitrogenase or leghemoglobin activity by nitrite [245,246], and inhibition of nitrogenase activity by the products of nitrogen fixation [247]. These factors include exposure of nodulated roots to nitrate [248,249,250]. Understanding these mechanisms is important for reducing crop fertilization and increasing the amount of plant nitrogen from nitrogen fixation. Inhibition of leghemoglobin synthesis and ammonia assimilating enzymes in nodules has been found to contribute to this process [215]. It has been determined that the decrease in nitrogenase activity in peas exposed to ammonium nitrate is attributed to a decline in leghemoglobin synthesis [244].
7. Mitigation Strategies
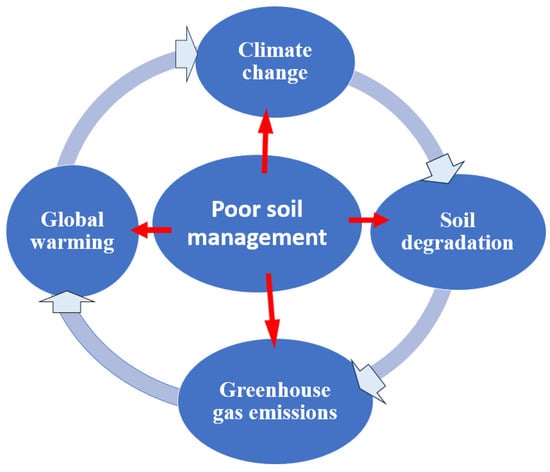
To mitigate climate change, it is crucial to find sustainable solutions for reducing nitrogen fertilizer use. One promising approach is through the utilization of Rhizobium–legume symbiosis. By harnessing this natural symbiosis, farmers can reduce their reliance on synthetic nitrogen fertilizers. This is significant because the production and application of nitrogen fertilizers contribute to greenhouse gas emissions, particularly nitrous oxide, which is a potent greenhouse gas (Figure 24). Selecting effective rhizobial strains: To enhance nodulation and nitrogen fixation efficiency, it is crucial to carefully select specific rhizobial strains. This can be accomplished by screening and choosing strains that have a higher compatibility with legume plants. Crop rotation and intercropping: Rotating legume crops with nonleguminous crops to break pest and disease cycles and promote nitrogen cycling in the soil. Intercropping legumes with other crops can enhance nutrient cycling and reduce the need for nitrogen fertilizers. Using cover crops: Planting cover crops, especially leguminous plants, during fallow periods can enhance soil health and increase the availability of nitrogen [260]. These cover crops can fix atmospheric nitrogen, making it accessible to subsequent crops [261]. Improving soil fertility: Employing practices such as organic matter addition, composting, and proper nutrient management to enhance soil fertility [262]. Proper soil management: Practice good soil management techniques to create a favorable environment for Rhizobium–legume symbiosis and microbial communities [266]. Maintain optimal soil pH and proper drainage, and avoid excessive use of nitrogen fertilizers, which can hinder nodulation and nitrogen fixation. Healthy soils are crucial for storing carbon and mitigating climate change. When managed sustainably, soils can sequester carbon and reduce greenhouse gas emissions. However, poor soil management and unsustainable agricultural practices can release carbon into the atmosphere, contributing to climate change (Figure 35). Improving farming practices: Adopting conservation agriculture practices, including reduced tillage, mulching, and water management techniques. These practices enhance soil structure, increase soil organic matter, and reduce nitrogen losses, maximizing the benefits of Rhizobium–legume symbiosis [270]. By adopting and implementing these improved farming practices, agricultural systems can become more resilient, efficient, and sustainable. This, in turn, helps to reduce the negative impacts of climate change on food production, ecosystems, and the environment. Integrated nutrient management: Combining Rhizobium inoculation with judicious application of mineral fertilizers to optimize nitrogen availability. This integrated approach ensures adequate nutrient supply while minimizing the use of synthetic fertilizers [272,273,274]. Rhizobial inoculant quality control: Verify the effectiveness of commercially available rhizobial inoculants through field trials or consultation with experts [275,276,277]. Use commercially available inoculants or isolate local strains proven to be effective in your specific region [278].