Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Hung-Yun Lin and Version 2 by Wendy Huang.
Thermal treatment is one of the common methods used in food processing to reduce microbial activity and control the presence of foodborne pathogens. During thermal processing, carbonization reactions are often observed in foods, which could result in the formation of carbon dots (CDs). Typical CDs are regarded as organic carbonization products with sizes less than 20 nm, exhibiting excitation-dependent fluorescence properties. They possess sp2/sp3 carbon skeletons and feature an abundance of functional groups and polymer chains within their structures. The surface of CDs is rich in hydrophilic compounds, including carboxyl, hydroxyl, and amine groups, contributing to their excellent water dispersibility.
- food-based carbon dots
- processed foods
- beverages
- raw food
- edible plants
- dietary compounds
1. Introduction
The synthesis of carbon nanotubes in 1985 and the subsequent isolation of graphene and carbon dots (CDs) in 2004 have been significant milestones in the advancement of carbon nanomaterials (CNMs) [1][2][1,2]. These materials possess exceptional properties that have revolutionized diverse industries, fostering scientific exploration and technological progress. For nearly 20 years, extensive research has been conducted on the physicochemical characteristics, synthesis methodologies, and wide-ranging applications of CNMs, placing them at the forefront of nanotechnology, energy, and materials science research [3]. Among them, the potential of CDs in biomedicine is gaining more and more interest due to their remarkable biocompatibility, light-emitting characteristics, drug delivery capabilities, immunomodulatory effects, and antimicrobial activity [3][4][5][6][3,4,5,6]. These characteristics position CDs as valuable tools in various clinical domains, including bioimaging, targeted drug delivery, pathogen control, and immunotherapy.
Typical CDs are regarded as organic carbonization products with sizes less than 20 nm, exhibiting excitation-dependent fluorescence properties [7]. They possess sp2/sp3 carbon skeletons and feature an abundance of functional groups and polymer chains within their structures [8]. The surface of CDs is rich in hydrophilic compounds, including carboxyl, hydroxyl, and amine groups, contributing to their excellent water dispersibility [9]. Before 2019, CDs could be further categorized based on their structure (Figure 1), including graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs), and carbonized polymer dots (CPDs) [10]. Among these, GQDs are characterized as two-dimensional materials with layered structures typically less than 20 nm in width, generally extending up to five layers (ca. 2.5 nm) [11]. Their primary planar structure consists of sp2 carbon hybrid arrangements, predominantly at the edges of graphene sheets or within interlayer defects [12]. Notably, GQDs exhibit distinct graphene lattice structures and a significant presence of chemical groups, particularly oxygen-containing functional groups, contributing to their unique properties, such as the quantum confinement effect and edge effect [13]. CQDs typically assume a spherical shape, with their carbon core primarily featuring excellent sp2 carbon crystallinity and sizes typically falling within the range of 1 to 10 nm [14]. The structural properties of CQDs enable them to exhibit intrinsic state luminescence and size-dependent quantum confinement effect. CNDs closely resemble CQDs in terms of size and shape [15]. They exhibit a higher degree of carbonization but typically lack a distinct lattice structure and do not manifest the quantum confinement effect related to particle size. The photoluminescence of CNDs stems from defects/surface states and subdomain states within the graphitic carbon core [16]. CPDs are produced through the carbonization of polymer compounds, with a relatively low degree of carbonization in their carbon core [10]. Their primary shared characteristic is the surface of the carbon core being enriched with outward-extending polymer functional groups, a result of passivation during the carbonization process. The photoluminescence of CPDs mainly originates from surface states, subdomain states, molecular states, and the crosslink-enhanced emission (CEE) effect [10].
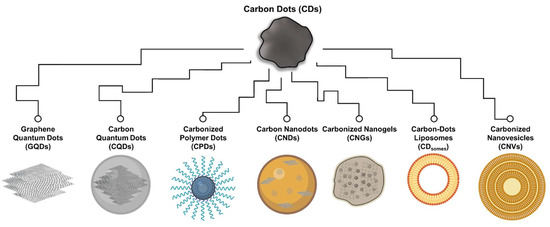
Figure 1. Classification of CDs family. Scheme illustrating the possible structure of various CDs, including graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs), carbonized polymer dots (CPDs), carbonized nanogels (CNGs), carbon-dot liposomes (CDsomes), and carbon nanovesicles (CNVs).
Recently, researchers have successfully synthesized and published several new classes of CDs, including carbonized nanogels (CNGs), carbon-dot liposomes (CDsomes), and carbon nanovesicles (CNVs), which share many characteristics in line with CDs but also exhibit distinct structural differences (Figure 1). CNGs, with sizes ranging from about 100 to 500 nm, are slightly larger than the typically defined in CDs [17][18][17,18]. They feature a carbonized structure comprising sp2-conjugated aromatic rings and sp3 polymer structures. Due to their graphene-like embedded polymer structure, CNGs can adopt either spherical or irregular-edged particle forms, displaying physical properties characterized by rheological properties similar to those of flexible polymer structures [17]. The photoluminescent characteristics of CNGs primarily stem from the π-conjugated macrocycle structure and edge chemical functional groups. Specifically, the reduction in vibration and rotation of subfluorophores within crosslinked gel structures is thought to trigger the CEE effect [17][18][17,18]. In addition, CDsomes are the carbonization products of long-chain hydrophobic compounds, whose carbon core structure comprises a conjugated benzene ring formed by a blend of sp2/sp3 carbon structure with oxygen-containing functional groups on the surface [19]. A significant characteristic of CDsomes is the asymmetric retention of the aliphatic chain from the precursor on their surface, giving rise to both hydrophilic and hydrophobic properties, rendering them amphipathic CDs with an approximate size of 5 nm. An even more significant and readily observable characteristic is the self-assembly of CDsomes in aqueous solutions, forming vesicles encased by a unilamellar bilayer of amphiphilic CDs. This structure bears a resemblance to liposomes and typically measures around 100 nm in size. The as-formed structure in water is attributed to amphiphilic interactions among the surface ligands, particularly including hydrophobic interactions between the oleate groups [19]. Based on their vesicle structure, CDsomes demonstrate excellent photostability, fusogenicity, and biocompatibility in aqueous solutions. Their excitation-dependent fluorescence properties can be attributed to the presence of polycyclic aromatic clusters, surface emissive traps, and edge defects present in amphipathic CDs of various sizes encapsulated within the vesicle structure [20]. CNVs, as the carbonization products of nonionic surfactants, also possess amphipathic CD characteristics with sizes typically around 3–5 nm [21][22][21,22]. Their vesicle structures, however, differ from CDsomes that self-assemble in aqueous solutions, as CNVs feature multilayered bilayer amphipathic CDs and exhibit structural characteristics resembling lipid nanoparticles. Nevertheless, the limited and potent evidence available does not allow for a clear differentiation in the mechanistic distinctions of photoluminescence characteristics. Hence, the classification of CDsomes and CNVs remains controversial.
The synthesis of CDs can be broadly classified into two main approaches: top-down and bottom-up strategies. The top-down approach involves the use of larger carbon substrates, such as graphite powers, graphite sheets, or carbon nanotubes (CNTs), which are prepared through methods like arc discharge, laser ablation, or electrochemical oxidation [23][24][23,24]. However, these methods are intricate and energy-intensive. In contrast, the bottom-up approach utilizes small molecular compounds, natural products, and even plant or food sources such as amino acids, organic acids, sugars, flavonoids, edible plants, or fruit juices [23]. By subjecting these materials to external heat, they undergo dehydration, condensation, and catalytic reactions, resulting in carbonization and the formation of CDs. The heat can be supplied through hydrothermal treatment (uniform heating in a solvent), microwave treatment (employing microwaves to facilitate reactions), or combustion (direct calcination) [3][23][3,23]. These strategies are known for their operational simplicity, scalability for mass production, and utilization of relatively low-cost instrumentation [3][4][5][6][3,4,5,6].
Interestingly, the heating conditions employed in these bottom-up strategies bear a resemblance to certain food processing techniques, such as stewing (prolonged closed heating at 60–120 °C for 1–5 h), frying/roasting (brief open heating at 100–300 °C for 5–60 min), and microwave cooking (600–1200 W for 1–10 min) [23][25][23,25]. Similarities in substrate abundance and synthetic strategies have led to investigations into the extraction of CDs from various processed foods using organic extraction methods [26][27][28][29][30][31][32][33][34][35][36][26,27,28,29,30,31,32,33,34,35,36]. Several studies have observed that substances like beer, instant coffee powder, roast duck, and even Traditional Chinese medicine derived from natural medicinal plants through decoction, baking, or roasting, have the potential to yield CDs [30][31][37][38][39][40][41][30,31,37,38,39,40,41]. In this resviearchw, CDs obtained using food-grade raw materials through food-like processing methods are regarded as food-based CDs. To date, a wide range of food-based CDs has been developed and extensively evaluated as highly biocompatible biomedical materials using diverse animal models.
2. CDs from Processed Food and Beverages
Thermal treatment is one of the common methods used in food processing to reduce microbial activity and control the presence of foodborne pathogens [42]. During thermal processing, carbonization reactions are often observed in foods, which could result in the formation of CDs [43][44][43,44]. Precursors typically undergo a series of carbonization steps, and certain studies offer time-dependent structural analyses illustrating this evolution. Initially, the precursor undergoes dehydration, leading to the aggregation and mild condensation of decomposition products, resulting in the formation of large-sized polymer supramolecular structures [44]. With the progression of heating, these polymer supramolecular structures contract due to ongoing intramolecular dehydration. This process is accompanied by the formation of carbon–carbon bonds and the development of aromatic clusters within the polymer. Once the density of clusters reaches a critical supersaturation point, the nucleation of the carbon core occurs. At this stage, aromatic clusters diffuse toward the particle surface to form nuclei, and the passivation of various functional groups occurs simultaneously [45]. To synthesize CPDs, CNGs, CDsomes, and CNVs, besides selecting suitable precursors, precise control of temperature and heating duration at this stage is crucial [10][17][18][19][20][21][22][10,17,18,19,20,21,22]. This control is essential for preserving effective functional groups and stabilizing the carbonized structure. In the case of other types of CDs exhibiting obvious/classical crystal lattices, polymer nanoparticles tend to dissipate or undergo transformation with increasing heating time [44]. This leads to a decrease in the polymer-to-dots ratio, resulting in smaller particle sizes and a narrower distribution of CDs. Numerous studies have provided insights into the extraction of CDs from thermally processed foods like stewed, roasted, or grilled lamb chops, beef, duck, chicken, eel, salmon, or hook snout carp [26][27][28][29][30][31][32][33][34][35][36][26,27,28,29,30,31,32,33,34,35,36]. Typically, the roasting process involves heating raw food in an oven at temperatures ranging from 100–350 °C for 5–60 min [46]. Grilling is a similar process that involves direct heating of the food on a metal grill. Stewing, on the other hand, entails slowly and gently heating raw food in a covered pot with a generous amount of broth at temperatures ranging from 60–80 °C for a duration of 3–5 h, or at 110–120 °C for 1–2 h [25]. For example, Geng et al. stewed beef in a pressure cooker at 117 °C and observed the yields of CDs as high as 0.05, 0.06, and 0.07% (v/v) after 30, 50, and 70 min of stewing, respectively [29]. The frying process, which involves submerging the raw food in vegetable or animal cooking oil, requires heat at temperatures around 150–200 °C for 10–15 min [25][47][25,47]. These heating conditions aligned with the synthesis requirements for most types of CDs in terms of temperature [23]. However, the allotted time is often insufficient to achieve complete carbonization of the raw materials, resulting in low yields of CDs [23][48][23,48]. The extracted CDs from processed foods are typically obtained using organic solvents, such as methanol or ethanol, and possess fluorescent properties [26][27][28][29][30][31][32][33][34][35][36][48][49][50][51][52][53][54][55][56][57][58][26,27,28,29,30,31,32,33,34,35,36,48,49,50,51,52,53,54,55,56,57,58]. Size-based separation techniques, such as dialysis and column chromatography, showed that food-based CDs exhibit sizes ranging from approximately 0.9 to 54.8 nm (Table 1). Interestingly, more complex processed foods, including pizza, burger meat, and canned foods, have also been reported to contain CDs with sizes ranging from 1.8 to 5.8 nm [49][50][51][49,50,51]. Other studies reported that commercially available beverages, including Nescafé, Coke, and Pepsi, also exhibit the presence of CDs, with particle sizes ranging from 0.9 to 39.1 nm [39][52][53][54][39,52,53,54]. These CDs in the beverages may originate from flavor enhancers that undergo high-temperature processing. Notably, caramel, one of the main ingredients in many commercial beverages, could also produce CDs. Studies showed that CDs in commercial beverages (2–5% w/v) were slightly higher than those found in other processed foods (typically ranging from 0.3% to 1.0% (w/v)) [26][27][28][29][30][31][32][33][34][35][36][48][49][50][51][52][53][54][55][56][57][58][59][26,27,28,29,30,31,32,33,34,35,36,48,49,50,51,52,53,54,55,56,57,58,59]. Furthermore, food-based CDs have also been identified in various fermented food products, including beer, bread, vinegar, soybean sauce, and tofu wastewater [37][38][48][55][56][57][58][59][37,38,48,55,56,57,58,59]. These CDs are believed to result from enzyme conversion reactions facilitated by probiotic microbes. The content of CDs in these foods typically ranges from 0.01% to 1.5%, similar to that found in heat-processed foods (Table 1). Interestingly, honey, a natural polysaccharide produced through the fermentation of plant nectars by microorganisms and enzymes in bees’ mouths, has also been found to contain CDs through dialysis and acetonitrile precipitation [60]. The in vivo synthesis of CDs through artificial induction strategies remains an unresolved difficulty. CD synthesis entails the decomposition, dehydration, and polymerization of organic molecules or polymers. Additionally, the verification of the hypothesis regarding CD induction in microorganisms is particularly challenging due to the constraints of in vitro assays in accurately simulating the intricate interplay of multienzymatic dehydration and carbon bond polymerization reactions involved [43][44][43,44]. As of now, no studies have discovered CDs produced by living organisms themselves. Notably, through a phytosynthesis process at 50 °C using plant leaf extracts in an in vitro environment with chitosan dissolved in an acidic solution, chitosan particles (ca. 10 nm) are synthesized [61][62][61,62]. While chitosan particles lack a crystal lattice and thus cannot be classified as CDs, they still illustrate the potential of bioenzyme catalysis in the production of novel CDs. The reaction is thought to potentially encompass the condensation and polymerization of several enzymes, including nitrate reductase, β-glucosidase, glycolytic enzymes, and aldolases [63]. Consequently, confirming this hypothesis continues to be a significant undertaking in the field of CD synthesis.Table 1.
Examples of CDs extracted from processed food and beverages.
| Food Groups | Food Source | Purification | Type/Size (nm) | Yield (%) | Quantum Yield (%) | Toxic Evaluation | Ref. |
|---|---|---|---|---|---|---|---|
| Complex processed foods | Pizza | Ethanol for 12 h, then dialyzed (0.5 kDa) | CNPs/2.6–4.1 | NA | 2.1 | In vitro >1 mg/mL, 6 h (Caco-2 cells) In vivo >100 mg/mL, 48 h (C. elegans) |
[49] |
| Burger meat (beef) | Ethanol for 12 h, then dialyzed (3.5 kDa) | CDs/0.9–54.8 | NA | 23.3 | In vitro >3.2 mg/mL, 12 h (MO cells) In vivo >3.2 mg/mL, 12 h (bean) |
[50] | |
| Canned yellow croaker | Ethanol for 12 h, then dialyzed (1 kDa) | CDs/1.8–5.8 | 0.3 (w/w) | 9.7 | In vitro >0.25 mg/mL, 12 h (HepG2 cells) |
[51] | |
| Commercial beverages | Nescafé® coffee | Size exclusion (Sephadex G-25) | CDs/3.0–6.0 | 2.0 (w/w) | 5.5 | In vitro >20 mg/mL, 24 h (CHO cells)/>1.5 mg/mL, 24 h (SMMC-7721 cells) In vivo >1000 mg/g, 56 h (guppy fish) |
[39] |
| ILLY® coffee | Dialyzed (14 kDa) | CQDs/2.0–7.0 | NA | NA | NA | [52] | |
| Cola | Size exclusion (Sephadex G-25) | CNPs/3.9–5.5 | 3.0 (w/v) | NA | In vitro >20.0 mg/mL, 24 h (CHO cells) In vivo >2000 mg/g, 24 h (mice) |
[53] | |
| Beverages | Size exclusion (Sephadex G-25) | CDs/2.8–39.1 | 2.0–5.0 (w/w) | 1.5–11.9 | In vitro >20 mg/mL, 24 h (CHO cells)/>10 mg/mL, 24 h (Tca-8113 cells) In vivo >40 mg/mL, 6 h (onion) |
[54] | |
| Fermented food products | Beer | Size exclusion (macroporous resin) | CDs/0.9–4.1 | NA | 1.4–3.9 | In vitro >5 mg/mL, 4 h (MC3T3-E1 cells) In vivo >2000 mg/kg, 24 h (mice) |
[37] |
| Tsingtao® beer | Size exclusion (Sephadex G-25) | CDs/1.0–5.0 | 1.2 (w/v) | 7.4 | In vitro >50 mg/mL, 48 h (MCF-7 cells) |
[38] | |
| Bread | Methanol for 10 min, then dialyzed (1 kDa) | CNPs/21.4–33.6 | NA | 1.2 | In vitro >2 µg/mL, 24 h (HeLa cells) |
[55] | |
| Bread | Methanol for 1 h, then dialyzed (1 kDa) | CNs/5.0–20.0 | NA | NA | In vitro >400 µg/mL, 48 h (hMSCs cells) |
[56] | |
| Breadcrumbs | Ethanol for 12 h, then dialyzed (3.5 kDa) | CDs/2.2–3.2 | 0.013 (w/v) | 1.8 | NA | [48] | |
| Vinegar | Size exclusion (macroporous resin) | CNPs/1.2–6.2 | 1.5 (w/v) | 5.7 | NA | [57] | |
| Vinegar | Ethanol for 12 h, then dialyzed (1 kDa) | CNPs/142.6–281.2 | NA | NA | In vitro 100 µg/mL, 24 h (Caco-2 cells) |
[58] | |
| Soybean sauce | Ethanol for 12 h, then dialyzed (1 kDa) | CNPs/298.5–398.2 | NA | NA | In vitro 100 µg/mL, 24 h (Caco-2 cells) |
[58] | |
| Tofu wastewater | Ultrasonic shock for 5 min, then centrifuged | CDs/2.0–10.0 | NA | NA | NA | [59] | |
| Flavor enhancers | Caramels | Methanol for 10 min, then dialyzed (1 kDa) | CNPs/2.8–5.8 | NA | 0.6 | NA | [55] |
| Jaggery | Methanol for 10 min, then dialyzed (1 kDa) | CNPs/12.8–27.8 | NA | 0.6 | In vitro >2 µg/mL, 24 h (HeLa cells) |
[55] | |
| Honey | Dialyzed for 48 h, treatment with acetonitrile, then lyophilized | CDs/1.7–4.7 | 1.5 (w/w) | 1.6 | NA | [60] |
NA—not available; CDs—carbon dots; CNPs—carbon nanoparticles; CNs—carbon nanostructures; CNTs—carbon nanotubes; CQDs—carbon quantum dots.
3. CDs Synthesized from Raw Food or Edible Plants
Raw foods, such as meat, vegetables, and fruits, are easily accessible sources of carbon compared to nature-derived chemicals. The bottom-up approach, encompassing dry burning, hydrothermal methods, or microwaving, is commonly employed to carbonize food and thereby generate CDs [23]. To obtain CDs from processed edible plants using the dry burning method, these materials are typically dried, and the resulting homogenized powders are heated in a muffle furnace at temperatures ranging from 180 °C to 400 °C for 1 to 6 h [64][65][66][64,65,66]. The original material undergoes proper oxidation during this heating process, facilitating the dehydration and polymerization of various carbon-containing functional groups [23][67][23,67]. Applying appropriate heating facilitates the thermal activation of reactions, supplying the necessary energy for chemical interactions between reactant molecules. This promotes the formation of sp2 hybridization and the development of a hexagonal carbon framework [68]. Furthermore, the heating-induced oxidative processes, driven by the electronegativity of oxygen atoms, lead to chemical bonding between carbon atoms, resulting in the introduction of various functional groups and heightened chemical reactivity [69]. However, prolonged heating during dry burning can lead to excessive carbonization, causing the dissipation of non-carbon atoms such as N, O, P, and S [70]. Hence, precise time control during the dry burning process is a critical factor in achieving a rich array of active functional groups on the surface of CDs. The dry-burning method, also known as powder carbonization, has received limited research attention, and the verification assays related to the graphite lattice are relatively inadequate [64][65][66][64,65,66]. For example, high-resolution transmission electron microscopy (HR-TEM) analysis of rose CDs fails to discern well-defined graphite lattice patterns (with lattice spacings of 0.246 nm or 0.335 nm), and X-ray diffraction (XRD) data is lacking for the validation of the graphite lattice (with 2θ values of 18.2° or 23.8°) [64]. Additionally, the elemental composition of CDs derived from Rhei radix rhizome and Phellodendri chinensis cortex reveals an excessively high proportion of oxygen elements (24.2% and 28.4%), implying that the synthesis of these CDs may have undergone excessive oxidation [65][66][65,66]. Ideal synthesis conditions for these CDs still require further optimization, implying potential progressiveness in the biomedical applications of CDs produced through powder carbonization. Hydrothermal carbonization is another method that has been used to produce CDs from raw foods or edible plants [71]. This method involves immersing the substance in a solvent, such as pure water, HCl, NaOH, or EtOH. The hydrothermal carbonization reaction is commonly enclosed in a Teflon autoclave, enabling a prolonged heating procedure at relatively high temperatures, typically ranging from 180–500 °C, for a duration of 2–12 h [71]. The pristine material in the solvent undergoes uniform heating, providing the necessary energy to facilitate oxidation, dehydration, cross-linking, and polymerization of functional groups [23][67][71][23,67,71]. This results in the formation of a carbon core predominantly based on the sp2 structure, such as graphene [68]. The hydrothermal carbon conversion process is gentler compared to dry burning, reducing the likelihood of excessive carbonization and retaining a higher degree of biologically functional groups on the surface of CDs [23][67][71][23,67,71]. Considering the main requirement of biosafety, pure water is used as the solvent for hydrothermal carbonization in food-derived CDs. Furthermore, microwave-assisted hydrothermal carbonization (MWAHTC) is another method that can be used to generate CDs from raw food or edible plant materials [72]. This method utilizes high microwave power to induce rapid internal vibrations in water molecules within the material, allowing for shorter processing times (5–60 min) to achieve efficient thermal energy transfer. The additional energy supplied is typically measured in watts (W), with power levels typically ranging from 70 to 1000 W. MWAHTC enables the conversion of carbon materials in a state that closely resembles the original food, and the common homogenization strategy involves simple chopping or grinding [72]. In comparison to conventional food processing methods, researchers found that dry burning, hydrothermal treatment, and microwave heating are more efficient in altering the properties of food by carbonizing its nutrient content into CDs [23]. In previous studies, researchers have used hydrothermal treatment or microwave heating processes in a wide variety of foods, including milk [73][74][75][73,74,75], fruits [76][77][78][79][80][81][82][76,77,78,79,80,81,82], and vegetables [83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] to produce CDs. These treatments have led to the production of CDs with sizes ranging from 0.5 to 31.1 nm (Table 2). Other studies have used natural flavor enhancers or natural sweeteners, such as guar gum and honey, for synthesizing CDs using MWAHTC or hydrothermal methods [112][113][112,113]. The use of the hydrothermal method has also been demonstrated in the production of CDs from probiotics that regulate intestinal flora to promote nutrient digestion/absorption and enhance immunity, including Escherichia coli, Bifidobacterium breve, Nannochloropsis oculata, and Bacillus cereus [114][115][116][114,115,116]. The size of the CDs generated from these probiotics was reported to range from 1.0 to 9.3 nm [114][115][116][114,115,116]. Notably, certain edible plants such as Phellodendri chinensis [65], Rhei Radix [66], forsythia [86], Chinese mugwort [88], ginkgo [90], green chiretta [91], Henna [92], rosemary [95], tea tree [97][98][97,98], ginger [99], and turmeric [103][104][103,104] are renowned for their therapeutic effects. These plants have gained popularity as valuable sources of carbon in the quest for CDs with medical properties (Table 2). Traditional Chinese medicine utilizes specific parts of these edible plants, including peels, fruits, seeds, roots, stems, and leaves, as raw materials [40]. These parts are collected and exposed to prolonged sunlight before being ground into powder. To transform them into therapeutic drugs, these medicinal plants undergo rigorous testing and processing, which includes heat treatment. Due to their richness in bioactive substances such as polysaccharides, polyphenols, and terpenoids, these plants constitute valuable resources for medicinal properties [117]. Interestingly, the process of transforming natural ingredients into CDs through heat treatment bears some resemblance to the traditional preparation of Chinese herbal medicine, where heat treatment is applied to raw materials [40][117][40,117]. Note that the manufacturing process of CDs employs modern molecular cooking techniques, utilizing high-purity molecules and precise processing conditions [40].Table 2.
Examples of CDs synthesized from raw food or edible plants.
| Food Groups | Food Source | Synthetic Method | Types/Size (nm) | Quantum Yield (%) | Toxic Evaluation | Potential Biomedical Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Raw meat | Lamb | Oven heating (280 °C for 15–45 min) | CDs/2.6–4.1 | 10 | In vitro >4 mg/mL, 24 h (PCl12 cells) |
Protein adsorption | [26] |
| Lamb | Oven heating (200–300 °C for 30 min), then extraction by ethanol for 24 h | CDs/1.7–2.8 | 6–45 | In vitro >2 mg/mL, 7 h (HepG2 cells) |
Scavenging ROS | [27] | |
| Beef | Oven heating (280 °C for 30 min), then extraction by ethanol for 30 min | CDs/1.0–4.0 | NA | In vitro >1 mg/mL, 12 h (NRK cells) |
Protein adsorption | [28] | |
| Beef broth | Oven heating (117 °C for 30–70 min), then extraction by ethanol for 40 min | CNPs/2.4–5.4 | 2.0–2.5 | In vitro >10 mg/mL, 24 h (NRK cells) |
Carrier for zinc | [29] | |
| Duck | Oven heating (200–300 °C for 30 min), then extraction by ethanol for 1 h | CDs/1.5–3.2 | 10.5–38.0 | In vitro >4 mg/mL, 36 h (PC12 cells) In vivo >15 mg/mL, 24 h (C. elegans) |
In vivo C. elegans bio-imaging | [30] | |
| Duck | Oven heating (170 °C for 1 h) then extract by ethanol for 1 h | CNPs/0.7–2.3 | 4.4 | NA | Protein adsorption | [31] | |
| Chicken | Oven heating (150–300 °C for 1 h) then extraction by ethanol for 36 h | CDs/1.5–20.4 | 6.5–17.9 | In vitro >4 mg/mL, 24 h (HepG2 cells) In vivo >2 g/kg, 20 h (mice) |
Dopamine sensing | [32] | |
| Pike eel | Oven heating (160–300 °C for 30 min), then extraction by ethanol for 24 h | CNs/1.8–4.3 | 80.2 | In vitro >20 mg/mL, 24 h (MC3T3-E1 cells) |
In vitro bio-imaging | [33] | |
| Atlantic salmon | Oven heating (200 °C for 10–60 min), then extraction by ethanol for 2 h | CQDs/1.9–4.1 | 2.2–12.1 | In vitro >6 mg/mL, 6 h (NRK cells) In vivo >2 g/kg, 24 h (mice) |
In vivo mice bio-imaging | [34] | |
| Mackerel | Oven heating (230 °C for 40 min), then extraction by ethanol for 2 h | CDs/0.9–3.5 | 12.0 | NA | Scavenging ROS | [35] | |
| Spanish Mackerel | Grill-heating (230 °C for 30 min) and then extraction by 10% methanol for 2 h | CDs/2.9–3.0 | NA | NA | Protein adsorption | [36] | |
| Processed food | Breadcrumbs | Oven heating (180 °C with cooking oil) then extraction by petroleum ether for overnight |
CDs/2.6–4.0 | 1.0 | NA | Protein adsorption | [48] |
| Flavor enhancers | Grounded spice of cinnamon, red chili, turmeric and black pepper | Hydrothermal (200 °C for 12 h) | CDs/10.3–15.0 | NA | In vitro >2.0 mg/mL, 24 h (HK2 cells) |
In vitro bio-imaging/Anticancer | [118] |
| Milk | Commercial cow milk | Hydrothermal (190–200 °C for 1–8 h) | CDs/ 0.5–4.0 |
NA | In vitro >0.4 mg/mL, 24 h (HT22 cells) |
Scavenging ROS | [73] |
| Commercial fat-free cow milk | Hydrothermal (180 °C for 2 h) | CDs/ 2.0–4.0 |
12 | In vitro >1 mg/mL, 24 h (U87 cells) |
In vitro bio-imaging | [74] | |
| Cow yogurt | Microwave (800 W for 30 min) | CDs/1.4–9.5 | 1.5 | In vitro >7.1 mg/mL, 100 h (MCF-7 and CoN cells) |
In vitro bio-imaging | [75] | |
| Fruits | Kiwi, Avocado, or Pear | Hydrothermal (200 °C for 12 h) | CDs/4.0–4.5 | 20–35 | In vitro >1.2 mg/mL, 72 h (HK-2 cells)/>2.2 mg/mL, 72 h (Caco-2 cells) In vivo >64 mg/mL, 80 h (zebra fish embryo) |
In vivo zebrafish bio-imaging/Anticancer | [76] |
| Mango | Hydrothermal (100 °C for 1 h, in H2SO4; 80 °C for 15 min, in H3PO4; 80 °C for 30 min, in H3PO4), then adjusted to pH 7.0 with NaOH | CNPs/5.0–10.0, 5.0–10.0, or 10.0–14.0 | 3.9, 1.6, or 0.5 | In vitro >5 mg/mL, 24 h (A549 cells) In vivo >5 mg/kg, 24 h (mice) |
In vivo mice bio-imaging | [77] | |
| Sapodilla fruits | Hydrothermal (100 °C for 1 h, in H2SO4; 80 °C for 15 min, in H3PO4; 80 °C for 30 min, in H3PO4), then adjusted to pH 7.0 with NaOH | CDs/1.6–2.2, 2.2–3.6, or 3.3–5.8 | 5.7, 7.9, or 5.2 | In vitro >300 µg/mL, 15 h (HeLa cells) |
In vivo bacterial/Fungal bio-imaging | [78] | |
| Cherry plum juice | Hydrothermal (200 °C for 20 h) | CDs/1.0–8.0 | NA | In vitro >500 µg/mL, 24 h (HepG2 cells) |
In vitro bio-imaging | [79] | |
| Lemon juice | Hydrothermal (120 °C for 3 h) | CQDs/2.0–4.5 | 9.0 | NA | In vivo plant bio-imaging (onion epidermal cells) | [80] | |
| Tomato juice | Hydrothermal (160 °C for 3 h) | CDs/2.4–3.6 | NA | In vitro >100 µg/mL, 96 h (A549, and Human dermal fibroblasts cells) |
Scavenging ROS | [81] | |
| Watermelon juice/Orange juice/Lemon juice/Cantaloupe juice/Red plum juice/Green plum juice/Carrot juice/Red pitaya juice/White pitaya juice | Hydrothermal (180 °C for 4 h) | CDs/1.6–5.6 | 13–25 | In vitro >1 mg/mL, 4 h (RAW 264.7 cells) In vivo >2 mg/kg, 5 h (zebrafish eleutheroembryo)/3.2 mg/kg, 6 h (zebrafish eleuthero-embryo) |
In vivo zebrafish bio-imaging (ROS sensing) | [82] | |
| Edible plants | Linseed (seeds) | Hydrothermal (180 °C for 12 h) | CDs/4.0–8.0 | 14.2 | In vitro >200 µg/mL, 24 h (MCF-7 cells) |
In vitro bio-imaging | [83] |
| Peanuts (seeds) | Hydrothermal (250 °C for 6 h) | CDs/2.0–8.0 | 7.9 | In vitro >1 mg/mL, 24 h (MCF-7 cells) |
In vitro bio-imaging | [84] | |
| Wheat bran (seeds) | Hydrothermal (180 °C for 3 h) | CDs/ca. 4.9 | 33.2 | In vitro >6 mg/mL, 24 h (SH-SY5Y cells) |
Drug carrier (amoxicillin; antibiotic) | [85] | |
| Forsythia (dried fruit powder) + Urea + Ethanolamine | Microwave (300 W for 2 min, repeat 3 times) | CQDs/1.8–3.6 | NA | NA | Antifungal | [86] | |
| Rose (flower petals) + thymol | Powder carbonization (180 °C for 6 h) and decorate with thymol | CDs/5.0–6.0 | NA | In vivo >10 mg/kg, 144 h (rats) |
Immuno-modulatory effect | [64] | |
| Phellodendri chinensis (Cortex) | Powder carbonization (400 °C for 1 h) | CDs/0.5–3.6 | 5.6 | In vitro >39 µg/mL, 24 h (L02, 293T, and RAW 264.7 cells) In vivo >0.86 mg/kg, 7 days (mice) |
Immuno-modulatory effect | [65] | |
| Cabbage (leaves) | Hydrothermal (140 °C for 5 h) | CQDs/2.0–8.0 | 16.5 | In vitro >700 µg/mL, 24 h (HaCaT cells) |
In vitro bio-imaging | [87] | |
| Chinese mugwort (leaves) | Purified fume particulate matter | CDs/3.0–7.0 | NA | In vitro >150 µg/mL, 24 h (HEK 293T cells) |
Antibacterial | [88] | |
| Coriander (leaves) | Hydrothermal (240 °C for 4 h) | CDs/1.5–3.0 | 6.48 | In vitro >1 mg/mL, 12 h (A549 and L-132 cells) |
Scavenging ROS/In vitro bio-imaging | [89] | |
| Ginkgo (leaves) | Hydrothermal (200 °C for 10 h) | CQDs/2.0–4.0 | 22.8 | NA | Disease detection in mouse serum | [90] | |
| Green chiretta (leaf extract) | Hydrothermal (160 °C for 8 h) |
CDs/8.0–11.0 | 15.1 | In vitro >700 µg/mL, 24 h (MCF-7 cells) |
Scavenging ROS/In vitro bio-imaging/Antibacterial/Anticancer | [91] | |
| Henna (leaves) | Hydrothermal (180 °C for 12 h) | CDs/2.7–7.8 | 28.7 (Rhodamine B) |
NA | Antibacterial/Anticancer drug sensing | [92] | |
| Holy basil (leaves) | Hydrothermal (180 °C for 4 h) | CDs/1.0–4.0 | 9.3 | In vitro >200 mg/mL, 24 h (MDA-MB-648 cells) |
In vitro bio-imaging | [93] | |
| Pakchoi (leaves) | Hydrothermal (150 °C for 12 h) | CDs/1.0–3.0 | 37.5 | In vitro >2 mg/mL, 24 h (HeLa cells) |
In vitro bio-imaging | [94] | |
| Rosemary (leaves) | Hydrothermal (140–200 °C for 6–12 h) | CDs/11.5–20.7 | NA | NA | Antibacterial | [95] | |
| Spinach (leaves) | Hydrothermal (150 °C for 6 h) | CDs/3.0–11.0 | 15.3 | In vitro >200 µg/mL, 24 h (A549 cells) In vivo >2 mg/mL, 24 h (mice) |
In vivo tumor imaging in mice | [96] | |
| Tea tree (leaves) | Hydrothermal (220 °C for 3 h) | CDs/1.7–5.0 | 4.9 | In vitro >4 mg/mL, 24 h (HepG2 cells) |
In vitro bio-imaging | [97] | |
| Tea tree /Osmanthus/Milk vetch (leaves) | Hydrothermal (200 °C for 2 h) | CDs/3.0–18.0 | NA | In vitro >1 mg/mL, 24 h (293T cells) |
Antibacterial | [98] | |
| Escallion (stem) | Hydrothermal (220 °C for 3 h) | CDs/ca. 4.22 | 10.5 | In vitro >200 µg/mL, 24 h (MCF-7 and K562 cells) |
In vitro bio-imaging | [99] | |
| Garlic (bulb) | Hydrothermal (180 °C for 10 h) | CDs/ca. 3.6 | 6.8 | NA | In vitro bio-imaging | [100] | |
| Ginger (rhizome) | Hydrothermal (300 °C for 20 min) | CDs/3.5–5.1 | 13.4 | In vitro >2.8 mg/mL, 24 h (A549, MDA-MB-231, and FL83B cells)/>1.4 mg/mL, 24 h (HeLa cells)/>0.4 mg/mL, 24 h (HepG2 cells) |
Anticancer | [101] | |
| Konjac (bulb) | Powder carbonization (470 °C for 1.5 h) | CDs/ca. 3.4 | 13.0 | In vitro >150 mg/mL, 12 h (HeLa cells) |
In vitro bio-imaging | [102] | |
| Rhei radix (rhizome) | Powder carbonization (350 °C for 1 h) | CDs/1.4–4.5 | NA | In vitro >200 µg/mL, 24 h (RAW 264.7 cells) |
Immuno-modulatory effect | [66] | |
| Turmeric (rhizome) | Hydrothermal (180 °C for 10 h) | CDs/1.5–4.0 | NA | In vitro >200 µg/mL, 24 h (PC3 cells) |
Antibacterial effects | [103] | |
| Turmeric (rhizome) + Ammonium persulfate | Hydrothermal (200 °C for 6 h) | CDs/9.4–11.8 | NA | In vitro >1 mg/mL, 72 h (L929 cells) |
Antibacterial effects/Scavenging ROS | [104] | |
| Yam (stem tuber) | Hydrothermal (200 °C for 2 h) | CDs/1.5–4.0 | 9.3 | NA | Anticancer drug sensing | [105] | |
| Beetroot (root) | Hydrothermal (160 °C for 8 h) |
CDs/<5.0 | 11.6 | In vitro >2.5 µg/mL, 24 h (HEK-293 cells) |
Anticancer/Scavenging ROS | [106] | |
| Carrot (root) | Hydrothermal (170 °C for 12 h) | CDs/ca. 2.3 | 7.6 | In vitro >2 mg/mL, 24 h (MCF-7 cells) |
Drug carrier (mitomycin; anticancer) | [107] | |
| Rose-heart radish (root) | Hydrothermal (180 °C for 3 h) | CDs/1.2–6.0 | 13.6 | In vitro >500 µg/mL, 3 h (SiHa cells) |
In vitro bio-imaging | [108] | |
| Sweet potato (root) | Hydrothermal (180 °C for 18 h) | CDs/2.5–5.5 | 8.6 | In vitro >150 µg/mL, 24 h (HeLa, HepG2 cells) |
In vitro bio-imaging | [109] | |
| Oyster mushroom (Sporocarp) | Hydrothermal (120 °C for 4 h; dissolved in 5% H2SO4) | CDs/5.0–18.0 | NA | In vitro >25 µg/mL, 24 h (HEK 293 cells) |
Antibacterial/Anticancer | [110] | |
| Water chestnut (bulb) + Onion (bulb) | Hydrothermal (180 °C for 4 h) | CDs/2.0–4.0 | 12.0 | In vitro >300 µg/mL, 24 h (T24 cells) |
In vivo bio-imaging and quantification of coenzyme A (pig liver) | [111] | |
| Natural flavor enhancers | Guar gum (Seed endosperm) | Microwave (400 W for 30 min) | CDs/19.2–31.1 | 7.5 | In vivo >1 mg/mL, 1 h (China rose leaf) |
In vivo plant bio-imaging (China rose leaf guard cells) | [112] |
| Honey + Garlic (bulb)+ Ammonia | Hydrothermal (200 °C for 6 h) | CQDs/4.0–13.0 | 4.2 | NA | Antibacterial | [113] |
NA—not available; CDs—carbon dots; CNPs—carbon nanoparticles; CNs—carbonaceous nanostructures; CQDs—carbon quantum dots.
4. CDs Synthesized from Dietary Compounds
Several studies have explored various carbonization strategies to obtain CDs from numerous edible compounds, such as food additives for food processing or health-promoting dietary compounds (Table 3) [119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146][147][148][149][150][151][119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151]. For example, CDs from alginate, curcumin, folicate, hesperidin, spermidine, and quercetin were obtained through a dry burning process (300–500 °C; 20–300 min) [132][133][134][135][136][137][138][139][140][141][132,133,134,135,136,137,138,139,140,141]. Another method, the so-called microwave method, has also been used to achieve the carbonization of these compounds in a shorter duration [72]. This method has been used to synthesize CDs from amino acids, proteins (i.e., casein and RNase A), and chitosan [132][133][134][135][136][137][138][139][140][141][132,133,134,135,136,137,138,139,140,141]. In other studies, researchers employed the hydrothermal method to enable the thermal carbon conversion of caffeic acid, fucoidan, and glycyrrhizic acid, resulting in the carbonization of CDs [142][143][144][145][146][147][148][149][150][151][142,143,144,145,146,147,148,149,150,151]. The identification of CDs derived from dietary compounds is typically performed using various analytical techniques, including Fourier-transform infrared spectroscopy (FT-IR), XRD, X-ray photoelectron spectroscopy (XPS), liquid chromatography-mass spectrometry (LC-Mass) and nuclear magnetic resonance spectroscopy (NMR) [152]. Several studies have revealed that utilizing dietary compounds in the carbonization reaction enables the inheritance of functional groups onto the surface of the carbon core throughout the heating process, resulting in the formation of novel CDs [119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146][147][148][149][150][151][119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151]. These functional groups, inherited to the CD surface, may then undergo thermal activation, leading to processes such as residue loss (e.g., decarboxylation and dehydrogenation), rearrangement (e.g., keto-enol tautomerism and conformational changes in aromatic rings), or fusion (e.g., carboxyl fusion and carboxylic acid-amine cross-reaction) [67][68][67,68]. The combinations of these newly generated functional groups during the carbonization process could potentially exhibit enhanced biological activities compared to the original bioactive compounds [119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146][147][148][149][150][151][119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151]. For example, Mao et al. demonstrated that the phenolic-like functional groups were produced during the carbonization reaction of alginate, which was not present in the pristine material [18]. Moreover, the carbonization process can modify the inherent physical properties of the resulting products by inheriting functional groups [20][21][119][130][20,21,119,130]. For instance, the one-step carbonization synthesis of curcumin, quercetin, sorbitan monolaurate (a common hydrophobic compound used as a food emulsifier), and triolein (main components in cooking oil) into CQDs [119], CNGs [130], CNVs [21], or CDsomes [20] was found to increase water dispersibility significantly. Additionally, combining with soluble components has demonstrated comparable effects. As an illustration, the co-carbonization of quercin and lysine notably improves the water solubility and biocompatibility of the resulting Qu/Lys-CNGs [17]. These enhancements in water dispersibility can be attributed to the distinctive combination of functional groups generated through thermal activation, leading to increased hydrophilicity and facilitating interactions with water molecules [17][119][130][17,119,130] or the formation of specific structures like nanovesicles [19][20][21][22][19,20,21,22]. These nanovesicles play a crucial role in the development of vesicular structures via amphiphilic CDs, with the hydrophilic end located on the outermost side of the CDs (i.e., CNVs and CDsomes).Table 3.
Examples of CDs synthesized from active compounds.
| Precursor | Synthetic Method | Type/Size (nm) | Yield (%) | Quantum Yield (%) | Toxic Evaluation | Potential Biomedical Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Ammonium citrate/Spermidine | Powder carbonization (180 °C for 2 h and 260 °C for 2 h) | CDs/3.8–5.4 | 50.8 | 2.8 | In vitro >50 mg/mL, 24 h (HEK-293T, MCF-7, A549, HeLa, and HaCaT cells) In vivo >50 mg/mL, 12 days (mice) |
Antibacterial/Wound healing | [153] |
| Citric acid + Diethyl-enetriamine | Powder carbonization (170 °C for 3 h in a nitrogen atmosphere) |
CDs/5.0–8.0 | NA | 25.5 | In vitro >100 μM, 24 h (A2780 cells) In vivo >100 μM, 14 days (mice) |
In vivo tumor image in mice/Drug carrier (cisplatin; anticancer) | [154] |
| Curcumin | Powder carbonization (180 °C for 2 h) |
CQDs/ 4.2–5.2 |
10.0–25.0 (w/w) | 0.3 | In vitro >50 mg/mL, 24 h (RD cells) In vivo >25 mg/kg, 15 days (mice) |
Antivirus | [119] |
| Curcumin | Powder carbonization (180 °C for 2 h) |
CQDs/ ca. 4.8 |
NA | NA | In vitro >100 mg/mL, 24 h (BHK-21 cells) |
Antivirus | [120] |
| Folic acid | Powder carbonization (140 °C for 6 h) |
CDs/1.0–1.6 | NA | NA | In vitro >200 μg/mL, 72 h (chondrocytes and macrophages) In vivo >2 mg/kg, 6 weeks (mice) |
Immuno-modulatory | [121] |
| Glutamic acid | Powder carbonization (210 °C for ~1 min) |
GQDs/3.4–5.9 | NA | 54.5 (NaOH) | In vitro >10 mg/mL, 1 h (MH-S cells) In vivo >25 mg/mL, 1 h (mice) |
In vivo bioimage in mice | [122] |
| Hesperidin | Powder carbonization (250 °C for 2 h) |
CPDs/46.7–60.1 | NA | NA | In vitro >500 μg/mL, 72 h (RD cells) In vivo >25 mg/kg, 9 days (mice) |
Antivirus | [123] |
| Spermidine | Powder carbonization (270 °C for 3 h) |
CQDs/ ca. 6.0 |
NA | 2.0–4.3 | In vitro >200 mg/mL, 24 h (RCK cells) |
Antibacterial | [124] |
| Spermidine | Powder carbonization (270 °C for 3 h) |
CQDs/ ca. 6.0 |
NA | 2.0–4.3 | In vitro >200 mg/mL, 24 h (RCK cells) |
Antivirus | [125] |
| Spermine + Dopamine | Powder carbonization (250 °C for 2 h) |
CQDs/ ca. 10.0 |
11.4 | 4.3 | In vitro >100 μg/mL 24 h (SIRC cells) In vivo >200 μg/mL, 14 days (rabbit) |
Antibacterial | [155] |
| Citric acid/boronic acids | Powder carbonization (250 °C for 0.5 h) and then mix with the boronic acid solution |
CQDs/5.4–7.0 | N.A | N.A | In vitro >600 μg/mL, 24 h (MOLT-4 cells) |
Antivirus | [156] |
| Lysine | Powder carbonization (270 °C for 3 h) | CNGs/120.0–510.0 | 66.5 | 8.1 | In vitro >50 μg/mL, 24 h (BHK-21 and Vero cells) In vivo >30 μg/mL, 7 days (chicken embryo) |
Antivirus | [126] |
| Lysine | Powder carbonization (270 °C for 3 h) |
CNGs/118.9–178.7 | 66.5 | 8.1 | In vitro >100 μg/mL, 24 h (HUVEC, RD, HepG2, HaCaT, and HEK-293T cells) |
Antibacterial | [127] |
| Lysine | Powder carbonization (270 °C for 3 h) |
CNGs/118.9–178.7 | 66.5 | 8.1 | In vivo 50 μg/mL, 96 h (zebrafish embryos)/10 μg/mL, 96 h (zebrafish eleutheroembryo)/0.5 μg/mL, 90 days (adult zebrafish)/2000 mg/kg, 48 h (guinea pigs)/2000 mg/kg, 72 h (rabbit)/2000 mg/kg, 14 days (rats) |
In vivo bioimage in zebrafish | [128] |
| Lysine or Arginine | Powder carbonization (240 °C for 3 h) |
CQDs/2.0–7.0 | NA | NA | In vitro >1 mg/mL, 24 h (NIH-3T3, BMSCs, and HUVECs cells) In vivo >2 mg/mL, 5 days (mice) |
Antibacterial/Scavenging ROS/Promoting tissue repair in mice | [129] |
| Quercetin | Powder carbonization (270 °C for 2 h) then dissolved in sodium phosphate buffer (pH 12) |
CNGs/326.9–423.3 | 78 | <1 | In vitro >1 mg/mL, 24 h (MDCK cells) In vivo >500 μg/mL, 14 days (mice) |
Antivirus | [130] |
| Quercetin + Lysine | Powder carbonization (270 °C for 3 h) |
CNGs/44.8–235.2 | 17.5 | 3.3 | In vitro >100 μg/mL, 24 h (SIRC cells) In vivo >50 μg/mL, 28 days (rabbit) |
Antibacterial/Scavenging ROS/Anti-inflammatory effects | [17] |
| Sodium alginate + Ammonium sulfite | Powder carbonization (180 °C for 3 h) |
CNGs/116.0–183.0 | 31.2 | 13.0 | In vitro >1 mg/mL, 24 h (MDCK cells) In vivo >500 μg/mL, 14 days (mice) |
Antivirus/Scavenging ROS/Anti-inflammatory effects | [157] |
| Sorbitan monolaurate | Powder carbonization (230 °C for 3 h) then dissolved in ethanol |
VCDs/390–430 | NA | NA | NA | Enzyme and nanomaterial carrier/Cholesterol detection in serum | [21] |
| Asparagine | Microwave (180 °C for 15 min) |
CDs/ca. 1.4 | NA | <1 | In vitro >800 μg/mL, 24 h (HeLa cells) |
In vitro bioimage | [131] |
| Casein (milk protein) | Microwave (450 W for 30 min; heating for 2 min and then pausing for 15 s) |
CDs/ca. 1.6 | NA | 18.7 | In vivo >200 μg/mL, 10 min (spinach leaf) |
In vivo plant bio-imaging (spinach guard and epidermal cells) | [132] |
| Chitosan | Microwave (700 W for 9.5 min) |
CDs/2.7–6.5 | 6.4 | 6.4 | NA | In vitro bioimage | [133] |
| Citric acid + Cysteine | Microwave (140 °C for 25 min) |
CQDs/0.9–1.0 | NA | 91.2 | In vivo ca. 1 mL/mice, 3 h (mice) |
Drug carrier (insulin)/In vivo glycemic control | [134] |
| Citric acid + Poly-ethyleneimine | Microwave (1150 W for 3 min) then mixed with locked nucleic acid (LNA) |
CDs/ca. 3.7 | NA | NA | In vitro >1 μg/mL, 3 days (KMM, BC3, BCP1, BCBL1, and BJAB cells) In vivo >50 μg/mice, 3 weeks (mice) |
Antivirus | [135] |
| Citric acid + RNase A enzyme | Microwave (700 W for 3–5 min) |
CDs/ca. 4.0 | NA | 24.2 | In vitro >3 mg/mL, 24 h (MGC-803 cells) In vivo >5 mg/mL, 24 h (mice) |
In vivo tumor imaging in mice | [136] |
| Citric acid + Tryptophan | Microwave (700 W for 3 min) |
CDs/ca. 2.6 | NA | 20.6 | In vitro >400 μg/mL, 24 h (MGC-803 cells) |
In vitro bioimage/Drug carrier (siRNA) | [137] |
| Citric acid + Urea | Microwave (800 W for 15 min) |
CDs/1.0–5.5 | NA | NA | NA | Antibacterial | [138] |
| Citric acid + Urea | SPMA (6 kW for 5 min) | GQDs/ 3.0–20.0 |
ca. 40 | NA | In vitro >50 μg/mL, 72 h (H171 cells) |
Antivirus | [139] |
| Citric acid + Urea | Microwave (650 W for 4–5 min), then powder carbonization (60 °C for 1 h) |
CDs/2.0–6.0 | NA | 36.0 | In vitro >100 μg/mL, 96 h, (HepG2 and HL-7702 cells) In vivo >500 μg/mL, 14 days (mice) |
Drug carrier (doxorubicin; anticancer)/In vivo tumor imaging in mice | [140] |
| Glucose + Arginine | Microwave (700 W for 10 min) |
CDs/1.0–7.0 | NA | 12.7 | In vitro >200 μg/mL, 24 h (MEFs cells) |
In vitro bioimage/Drug carrier (circular DNA)/Chondrogenic differentiation | [141] |
| Microcrystalline cellulose | Alkaline hydrolysis (90 °C for 2 h), then infrared-assisted heating (125 °C for 6 h) | CQDs/6.7–12.5 | NA | NA | NA | Antibacterial/Anticancer | [148] |
| Boronic acid derivatives | Hydrothermal (160 °C for 8 h) |
CQDs/8.9–9.5 | NA | 0.05 | In vitro >100 μg/mL, 8 h (Huh-7 cells) |
Antivirus | [158] |
| Ciprofloxacin (antibiotic) | Hydrothermal (200 °C for 4 h) |
CDs/4.7–6.8 | NA | 25.3 | NA | Antibacterial | [159] |
| Citric acid + amino acid (Arg, Cys, Glu, Gly, His, Leu, Phe, and Tyr) | Hydrothermal (180 °C for 12 h; dissolved in formamide) |
CDs/3.0–6.0 | NA | 25.5–62.1 | In vitro >100 μg/mL, 24 h (HeLa cells) |
In vitro bioimage | [160] |
| Citric acid + Curcumin | Hydrothermal (180 °C for 1 h) |
CQDs/1.2–1.8 | NA | 3.6 | In vitro >250 μg/mL, 18 h (RAW 264.7 cells) |
Antivirus | [161] |
| Citric acid + Branched poly-ethyleneimine | Hydrothermal (200 °C for 12 h) |
CQDs/2.0–8.0 | NA | NA | In vitro >500 μg/mL, 72 h (L929 cells) |
Antibacterial | [162] |
| Citric acid + Curcumin | Hydrothermal (180 °C for 24 h) |
CDs/1.5–2.5 | NA | 30 | In vitro >250 μg/mL, 48 h (RAW 264.7, HK-2, and HPMCs cells) |
Antibacterial | [163] |
| Citric acid + Ethyl-enediamine/ampicillin (antibiotic) | Hydrothermal (250 °C for 4 h) coupled with ampicillin conjugation | CDs/ca. 34.0–54.0 | 60 | 19 | In vitro >200 μg/mL, 24 h (HeLa cells) |
Antibacterial | [164] |
| Vit C + PEG-diamine | Hydrothermal (180 °C for 1 h) |
CDs/4.7 | NA | NA | In vitro >250 μg/mL, 48 h (PK-15 and MARC-145 cells) |
Antivirus | [165] |
| Caffeic acid | Hydrothermal (200 °C for 6 h) |
CQDs/1.5–2.5 | 10.2 | NA | In vitro >10 mg/mL, 12 h (HeLa cells) |
Antibacterial/Antivirus | [142] |
| Carrageenan or Pullulan | Alkaline hydrolysis (90 °C for 2 h), then hydrothermal (210 °C for 6 h) |
CQDs/ ca. 3.1 or ca. 4.2 |
NA | NA | In vitro >1000 or >500 μg/mL, 24 h (Vero E6 cells) |
Antivirus/Anticancer | [143] |
| Chlorogenic acid + Caffeic acid + Quinic acid | Hydrothermal (230 °C for 2 h) |
CQDs/5.0–10.0 | NA | NA | In vitro >100 μg/mL, 24 h (L02 cells) In vivo >200 mg/kg, 90 min (mice) |
Anticancer/GSH oxidase-like activity/Scavenging ROS | [52] |
| Folic acid | Hydrothermal (180 °C for 2 h) |
CDs/3.0–11.0 | NA | 23.0 | In vitro >1 mg/mL, 3 h (U87 cells) |
In vitro bioimage | [144] |
| Fucoidan | Hydrothermal (200 °C for 12 h) |
CDs/4.0–10.0 | NA | NA | In vitro >1 mg/mL, 3 h (MC3T3-E1 cells) |
Antibacterial | [145] |
| Glucose, Vit C, or Fructose | Hydrothermal (200 °C for 12 h) |
CDs/ca. 9.0–10.0 | 34/56/29 (w/w) | 1.8/1.5/0.3 | In vitro >1000/>250/<1 μg/mL, 96 h (HeLa cells) |
Drug carrier (doxorubicin) | [146] |
| Glucose + Ethylenediamine | Hydrothermal (200 °C for 4 h) |
CDs/1.0–3.0 | NA | NA | In vivo >2.5 mg/mL, 3 h (zebrafish embryos)/>1.5 mg/mL, 10 h (zebrafish eleuthero-embryos) |
In vivo bio-imaging in zebrafish embryos and eleuthero-embryos | [166] |
| Glucose + Glutamic acid | Hydrothermal (125 °C for 30 min, then 200 °C for 20 min; dissolved in NaOH) |
CDs/ca. 2.0 | 29.8 | NA | In vitro >1000 μg/mL, 48 h (HeLa cells) |
Drug and fluorescent dye carrier (doxorubicin; anticancer)/In vitro bioimage | [167] |
| Glucose + Aspartic acid | Hydrothermal (125 °C for 30 min, then 200 °C for 20 min; dissolved in NaOH) |
CDs/1.8–2.7 | 34.5 | 7.5 | In vitro >500 μg/mL, 48 h (L929 and C6 cells) In vivo >200 mg/kg, 90 min (mice) |
In vivo tumor image in mice | [168] |
| Glycyrrhizic acid | Hydrothermal (180 °C for 7 h; dissolved in NaOH) |
CQDs/ ca. 11.4 |
NA | 1.4 | In vitro >450 μg/mL, 48 h (MRC 145 cells) In vivo >200 mg/kg, 90 min (mice) |
Antivirus | [143] |
| Sorbitol + Ethyl-enediamine | Hydrothermal (180 °C for 5 h) |
CDs/ca. 5.0 | NA | 8.9 | In vitro >1000 μg/mL, 24 h (MCF-7 cells) |
In vitro bioimage | [160] |
| Vitamin C | Hydrothermal (180 °C for 4 h) |
CDs/ca. 9.0 | NA | NA | In vitro >1 mg/mL, 48 h (NIH-3T3 cells) In vivo >1 mg/mL, 48 h (fungus) |
Fluorescent dye carrier/In vivo bioimaging in fungus Candida albicans | [149] |
| Triolein | Hydrothermal (220 °C for 3 days), then dissolved in NaOH |
CDsomes/80.0–100.0 | ca. 30 | 4.1 | In vitro >300 μg/mL, 24 h (HaCaT cells) In vivo >100 μg/mL, 12 days (mice) |
Antibacterial/Controllable ROS induction/Wound healing | [150] |
| Triolein | Hydrothermal (220 °C for 3 days), then dissolved in NaOH |
CDsomes/80.0–100.0 | NA | 1.0 | In vitro >300 μg/mL, 48 h (HeLa cells) |
In vitro bioimage | [20] |
| Triolein | Hydrothermal (220 °C for 3 days), then dissolved in NaOH |
CDsomes/80.0–100.0 | 68 | NA | In vitro >400 μg/mL, 24 h (NIH-3T3 cells) |
Anticancer/Controlable ROS induction | [151] |
| Citric acid + Glutathione | Oil bath (200 °C) | CDs/2.5–3.0 | NA | 80.3 | In vitro >3 mg/mL, 24 h (A549 cells) |
In vitro bioimage | [169] |
| Vitamin C | Electrolysis (0.1 A for 3 weeks) |
CDs/3.0–6.0 | NA | ca. 30 | NA | Antibacterial/Antifungal | [170] |
NA—not available; CDs—carbon dots; CDsomes—carbon dot liposomes; CNGs—carbon nanogels; CNPs—carbon nanoparticles; CPDs—carbon polymer dots; CQDs—carbon quantum dots; GQDs—graphene quantum dots; VCDs—vesicle-like carbon dots. SPMA—solid-phase microwave-assisted technique.
