Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Rita Xu and Version 2 by Rita Xu.
Rheumatoid arthritis (RA) is an invalidating chronic autoimmune disorder characterized by joint inflammation and progressive bone damage. Dietary intervention is an important component in the treatment of RA to mitigate oxidative stress, a major pathogenic driver of the disease. Alongside traditional sources of antioxidants, microalgae—a diverse group of photosynthetic prokaryotes and eukaryotes—are emerging as anti-inflammatory and immunomodulatory food supplements.
- photosynthesis
- polyunsaturated fatty acids
- carotenoids
- oxylipins
- xanthophylls
- antioxidants
- functional foods
- synthetic biology
1. Introduction
Chronic inflammation is a defining feature of autoimmune diseases, a group of conditions in which immunological self-tolerance is disturbed due to the recognition of autoantigens by immune cells. Rheumatoid arthritis (RA), the most common chronic inflammatory arthropathy [1][2], is a systemic autoimmune disorder affecting the synovial joints, with a higher incidence in women [3]. RA displays a complex pathophysiology involving the upregulation of pro-inflammatory mediators (interleukins, ILs) and enhanced production of reactive oxygen species (ROS) [4][5]. Both genetic and modifiable lifestyle factors contribute to the risk of RA predisposition [6][7], with diet highly influencing disease activity [8][9]. In particular, a high antioxidant intake is known to reduce onset risk and to ameliorate the clinical course of the disease [10], therefore, the identification of new sources of antioxidant and anti-inflammatory molecules is of high clinical relevance.
Microalgae are photosynthetic prokaryotes and eukaryotes adapted to diverse environments, including extreme habitats [11][12], which are consumed in human nutrition as sources of proteins and other bioactive compounds [13][14][15][16][17]. Several species are non-toxic producers of essential vitamins, lipids, and pigments of therapeutic value [18][19][20][21][22], which could be employed as complementary agents in the management of chronic inflammatory diseases. Moreover, the fast life cycle and light-powered autotrophic metabolism of microalgae allows for large-scale cultivation with lower inputs compared with heterotrophic microorganisms [23].
Pathogenesis and Mediators of Rheumatoid Arthritis
Although the exact etiology of RA remains unknown, the balance between immune cells and the production of inflammatory ILs in the connective tissue that lines the joint capsule (synovium) is altered in the disease onset and progression [4][5]. The healthy synovium consists of a thin lining layer of fibroblasts covering a connective tissue surrounded by blood vessels and enriched in fibroblasts, and innate and adaptive immune cells: the sub-lining layer [24] (Figure 1A). In RA, the lining layer is hyperplastic while the sub-lining layer is infiltrated with B-cells, monocyte-derived macrophages, autoantibody-secreting plasma cells, and differentiated cytotoxic CD4+ T-cells involved in the breakdown of tissue tolerance [25][26][27]. The release of pro-inflammatory ILs by monocyte-derived M1 macrophages, and osteoclast activation cause progressive bone resorption [28], and autoantibodies produced by differentiated plasma cells further contribute to joint damage [29].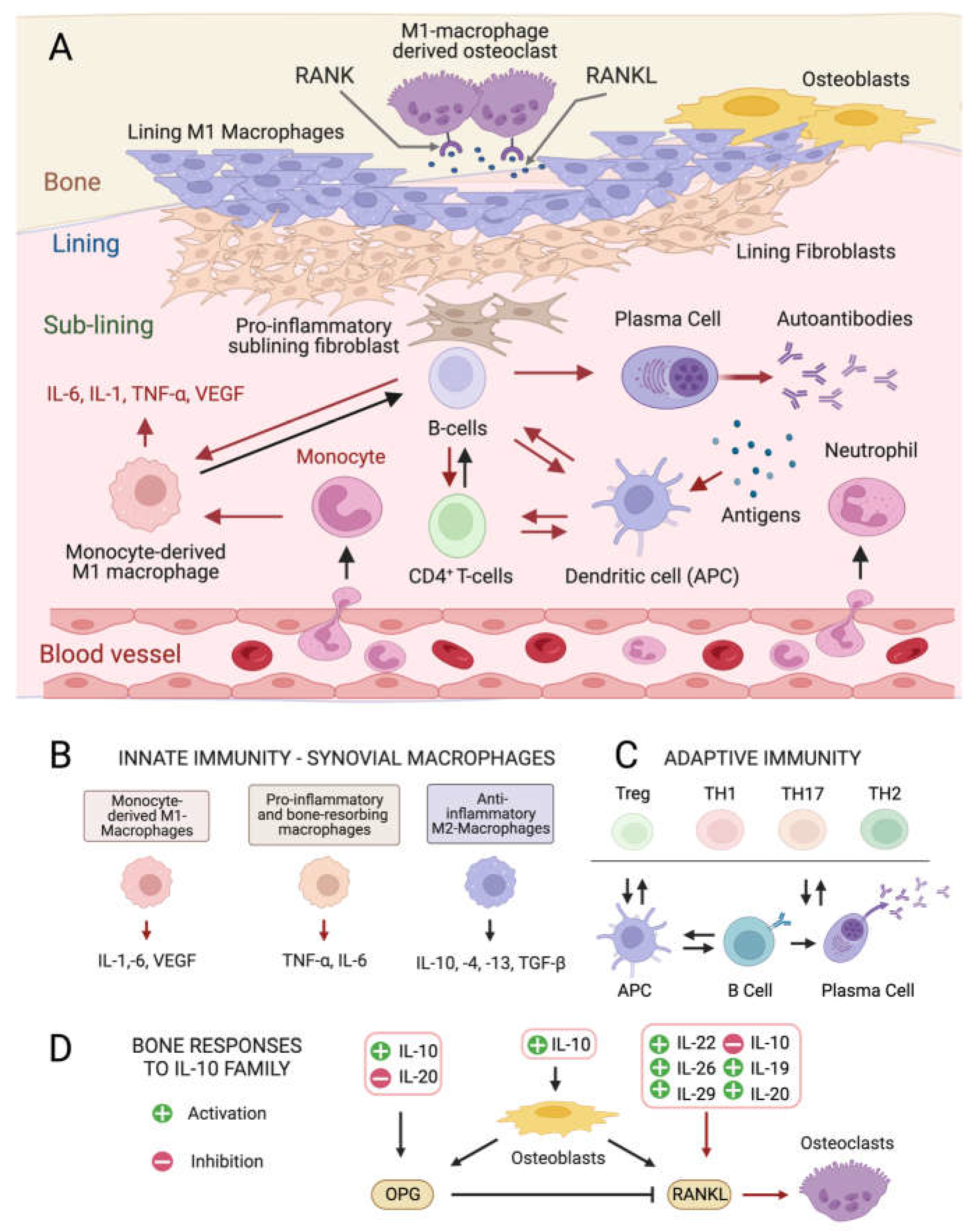
Figure 1. Cellular composition of the synovial membrane, its interaction with bone tissue and immune cell types, and related mediators involved in inflamed RA joints. (A) In healthy conditions, the synovium consist of a thin lining layer of lining fibroblasts in association with lining M1 directly exposed to the bone tissue. The underlying sub-lining layer is a connective tissue enriched in blood vessels, adipocytes, fibroblasts, and both innate and adaptive immune cells. The inflamed RA synovium is characterized by a hyperplastic lining layer surrounded by proinflammatory sub-lining fibroblasts and a massive infiltration of B-cells, monocyte-derived macrophages, autoantibody-secreting plasma cells, and differentiated cytotoxic effector memory CD4+ T-cells in the sub-lining layer. The secretion of pro-inflammatory interleukins (IL-1: interleukin 1; IL-6: interleukin 6; TNF- α: tumor necrosis factor-alpha) by activated immune cells stimulates the production of the soluble cytokine Mediator Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL), which binds to its receptor RANK on monocytes and macrophages causing their differentiation into bone-resorbing osteoclasts. Red arrows indicate pro-inflammatory processes. (B) Interleukin (IL) isoforms produced by different types of synovial innate immune cells and macrophages (IL-6: interleukin 6; IL-1: interleukin 1; TNF-α: tumor necrosis factor-alpha; TNF-β: tumor necrosis factor-beta; VEGF: vascular endothelial growth factor). (C) Differentiation and interconversion of adaptive immune cells (Treg: regulatory T cells; TH1: T helper 1 cells; TH 17: T helper 17 cells; TH2: T helper 2 cells; B cells; APCs: antigen-presenting cells). (D) Effects of secreted ILs on bone-remodeling processes depending on the osteoprotegerin (OPG)–RANKL axis, which regulates the differentiation of osteoclasts in bone-resorbing osteoclasts.
2. Anti-Inflammatory and Immunomodulatory Metabolites from Microalgae
2.1. Carotenes and Xanthophylls
Like bacteria, fungi, and plants, microalgae synthetize C40 lipophilic pigments consisting of a polyene chain of conjugated double bonds (Figure 2) with terminally linked ionone rings known as carotenoids [37][38]. β-carotene (Figure 2B)—a structural element of photosystems [39]—and the antioxidant lycopene (Figure 2A) are anti-inflammatory carotenes [40][41] usually introduced in the diet with carrots (Daucus carota) and tomatoes (Solanum lycopersicum), respectively, although present also in microalgae [42]. Xanthophylls are oxygenated carotenoids containing hydroxyl and ketone groups in the ionone rings, which serve different functions in phototrophs. The non-ketolated xanthophyll lutein (Figure 2C) participates in light-harvesting and photoprotection, while the ketocarotenoid astaxanthin (ASTX, Figure 2D) scavenges harmful ROS generated by photosynthetic electron transport under excess light [43]. Lutein is an anti-inflammatory carotenoid [44] abundantly found in green leafy vegetables and egg yolk, while ASTX is a potent antioxidant uniquely synthetized by a few microalgal species. Abiotic stresses induce a hypercarotenogenic response in several chlorophytes, including the halophile Dunaliella salina (Chlorophyceae), which overaccumulates β-carotene in lipid bodies (plastoglobules) inside the chloroplast [45], and in the freshwater species Haematococcus pluvialis (Haematococcaceae), which forms haematocysts filled with ASTX-rich cytoplasmic lipid droplets [46]. Other microalgal xanthophylls with anti-inflammatory and immunomodulatory properties are fucoxanthin and diatoxanthin produced by several diatoms (stramenopiles) and by the haptophyte Tisochrysis lutea (Coccolithophyceae) (Figure 2E,F) [47][48]. As discussed in the following paragraphs, carotenoids appear to interfere in all major pro-inflammatory pathways implicated in the onset and progression of RA.Astaxanthin: The Red Gold of Algae
With recognized safety for human consumption [49], approved Novel Food status [50], and an established role in promoting bone homeostasis in degenerative skeletal diseases [51], ASTX is the microalgal pigment of highest biopharmaceutical value. Clinical studies have shown that ASTX intake reduces the levels of systemic inflammatory biomarkers [52][53] and potentiates the pain-relieving effect of anti-inflammatory therapies [54]. The pharmacological effects of ASTX derive from its strong antioxidant-activity mediated via ROS quenching [55] (Figure 2G, top panel) and direct free radical scavenging [56][57] (Figure 2G, bottom panel). This amphipathic molecule is symmetrically arranged within the lipid bilayer [58], thus exerting antioxidant activity on both intra- and extracellular environments. Notably, the higher number of hydroxyl groups compared with other carotenoids confers to ASTX superior ROS-detoxifying capacity [59]. Early studies showed that ASTX suppressed ROS production [60][61][62][63] and secretion of pro-inflammatory ILs by cultured human-activated monocytes [64]. Moreover ASTX stimulated the expression of ROS-scavenging enzymes in chondrocytes challenged with IL-1β [65], and inhibited pro-inflammatory and osteoclastogenic gene expression in macrophages challenged with RANKL [66]. Lastly, the administration of ASTX promoted cartilage health in animal models of arthritis and osteoasthritis [67][68][69].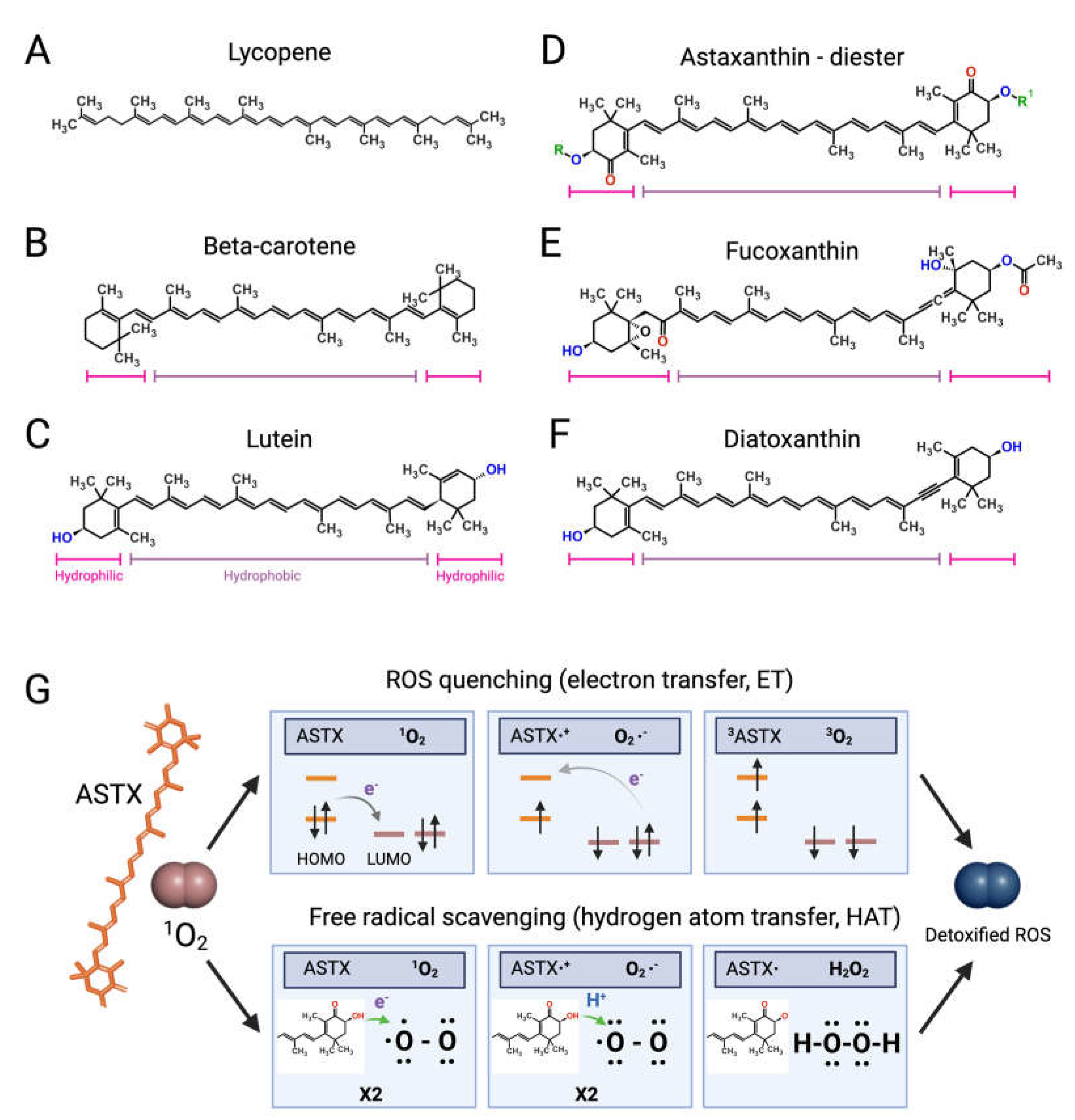
Figure 2. Structures of microalgal carotenoids and ROS detoxification mechanisms of astaxanthin. Lycopene (A), beta-carotene (B), lutein (C), astaxanthin (ASTX, (D)), fucoxanthin (E), and diatoxanthin (F). Pink and purple bars indicate the hydrophobic and hydrophilic regions of the molecules, respectively; in red, the oxygen of the keto groups, and in blue, the oxygen of the carboxylic groups; in green, the R and R’ functional groups of astaxanthin. Panel (G) outlines the two main routes of ASTX-mediated singlet molecule oxygen (1O2) detoxification. The top pathway is based on an electron transfer process involving: (i) the formation of a weakly bound ASTX-1O2 complex followed by direct electron transfer from the highest occupied molecular orbital (HOMO) of ASTX to the lowest unoccupied molecular orbital (LUMO) of singlet oxygen (1O2), and the formation of radicals; (ii) a reverse reaction restoring the electron distribution between the two molecules. The overall process converts 1O2 to its triplet unreactive form (3O2) upon spin inversion, while ASTX is restored from 3ASTX via internal conversion. The bottom pathway shows the free radical scavenging activity based on a two-step transfer involving both an electron and proton (H+) from ASTX to 1O2. The formed hydrogen peroxide is readily removed by peroxidase enzymes while ASTX is spontaneously restored by ascorbate. These mechanisms of action are iterative, meaning that a single ASTX molecule can perform multiple ROS detoxification cycles.
2.2. Anti-Inflammatory Mechanisms of Action of Astaxanthin and Other Carotenoids
2.2.1. NF-κB Pathway
ASTX and β-carotene interfere with the NF-κB pathway blocking the translocation of the NF-κB transcription factor to the nucleus, thereby suppressing ROS and pro-inflammatory gene expression. This effect is likely mediated through targeting the Inhibitor of the NF-κB γ subunit (IKK-γ) of the IkB kinase complex [81][82][83][84]. This prevents the phosphorylation and subsequent proteasome-mediated degradation of the IkBα binding factor, which abolishes the release of NF-κB [30]. A similar inhibitory effect has been proposed for fucoxanthin and diatoxanthin [85][86]. The Mitogen- and Stress-activated protein Kinase-1 (MSK1) is a nucleus-localized factor, which activates the NF-κB pathway [87] and the transcriptional regulator cAMP-responsive Element-Binding Protein (CREB) [88]. Phosphorylated CREB binds CREB-Responsive Elements (CRE) promoting pro-inflammatory gene expression [89]. These events are suppressed by ASTX, which inhibits MSK1 autophosphorylation [90]. Lastly, in silico simulations suggested that ASTX and β-carotene extracellularly interact with IL-6 and TNF-α, preventing their binding to membrane receptors [91]. ASTX may also interact with the NF-κB-Inducing Kinase (NIK) and block the phosphorylation of the IKK-α subunit of the IkB α kinase complex, suppressing the NF-κB pathway [92].2.2.2. JAK2/STAT3 and JNK/p38 MAPK Pathways
β-carotene and ASTX further modulate the pro-inflammatory pathways mediated by the JNK/p38 MAPK [93] and JAK2/STAT3 kinases [84][94], the latter responding to IL-6 in the pathogenesis of RA and osteoarthritis [31][95]. Phosphorylation of STAT3 dimers by JAK2 induces nuclear translocation and the differentiation of CD4+ T cells into the highly reactive T helper 17 (Th17) cell type [96]. TNFs and IL-1 activate the JNK/p38 MAPK pathway starting a phosphorylation cascade ending with JNK/p38 MAPK nuclear translocation [97], and phosphorylation of pro-inflammatory transcription factors (ELK1, MEF2, ATF2, and STAT1) [98] and of the MAPK-activated kinase 2 (MK2), which, in turn, targets the tristetrapolin (TTP) factor, promoting stabilization of IL mRNAs [99]. These oxidant-sensitive inflammatory pathways are also modulated by lutein [100], as reported using extracts enriched in this xanthophyll from different species of the chlorophyte genus Tetraselmis (Chlorodendrophyceae) [101].2.2.3. Other Pro-Inflammatory Pathways Targeted by Microalgal Carotenoids
Mitochondrial disfunction is a key pathogenic driver in RA [102], and ASTX was reported to attenuate organellar ROS production in human chondrocytes treated with IL-1β [69]. In addition to suppressing pro-inflammatory pathways, ASTX is also suggested to promote cartilage homeostasis via the transcriptional regulator nuclear factor-erythroid 2-related factor 2 (Nrf2) [68][93]. ASTX is suggested to stabilize and promote the nuclear translocation of Nfr2, which binds so-called antioxidant response elements (AREs), enhancing the expression of anti-inflammatory and ROS-detoxifying genes [103][104].2.3. Lipids and Their Derivatives
A hallmark of RA is the altered fatty acid profile of the synovium [105][106], while the intake of polyunsaturated fatty acids (PUFAs) correlates with joint health and mitigates the risk of RA onset [107]. Microalgal mass cultivation is a more sustainable way to derive functional lipids compared with cold water fish [108][109][110][111][112]. Phytoplankton occupies the lowest trophic level in oceans and freshwater basins, representing the primary PUFAs producer in aquatic food webs [113]. Global warning and ocean acidification are predicted to affect phytoplankton ecology [114][115], thus reducing PUFAs availability to higher trophic levels and, eventually, putting at risk the supply for human nutrition [116]. Moreover, upon stress acclimation, microalgae synthetise a wider range of anti-inflammatory and immunomodulatory lipids compared to animals [117][118][119][120][121].2.3.1. Long-Chain Polyunsaturated Fatty Acids
Several microalgae accumulate very long-chain PUFAs [122][123], including the omega-3 (ω-3, n-3) PUFAs [124] α-linolenic (18:3), docosahexaenoic (DHA, 22:6, n-3, Figure 3A), docosapentaenoic (DPA, n-3, 22:5, Figure 3B), and eicosapentaenoic (EPA, 20:5, n-3, Figure 3C) acids but also ω-6 PUFAs like arachidonic (ARA, 20:4, n-6, Figure 3H), γ-linolenic (18:3), linoleic (18:2), and dihomo-γ-linolenic (DGLA, 20:3, n-6, Figure 3D) acids. These molecules are biosynthetic precursors of anti-inflammatory signaling molecules and interfere with pro-inflammatory pathways [125][126].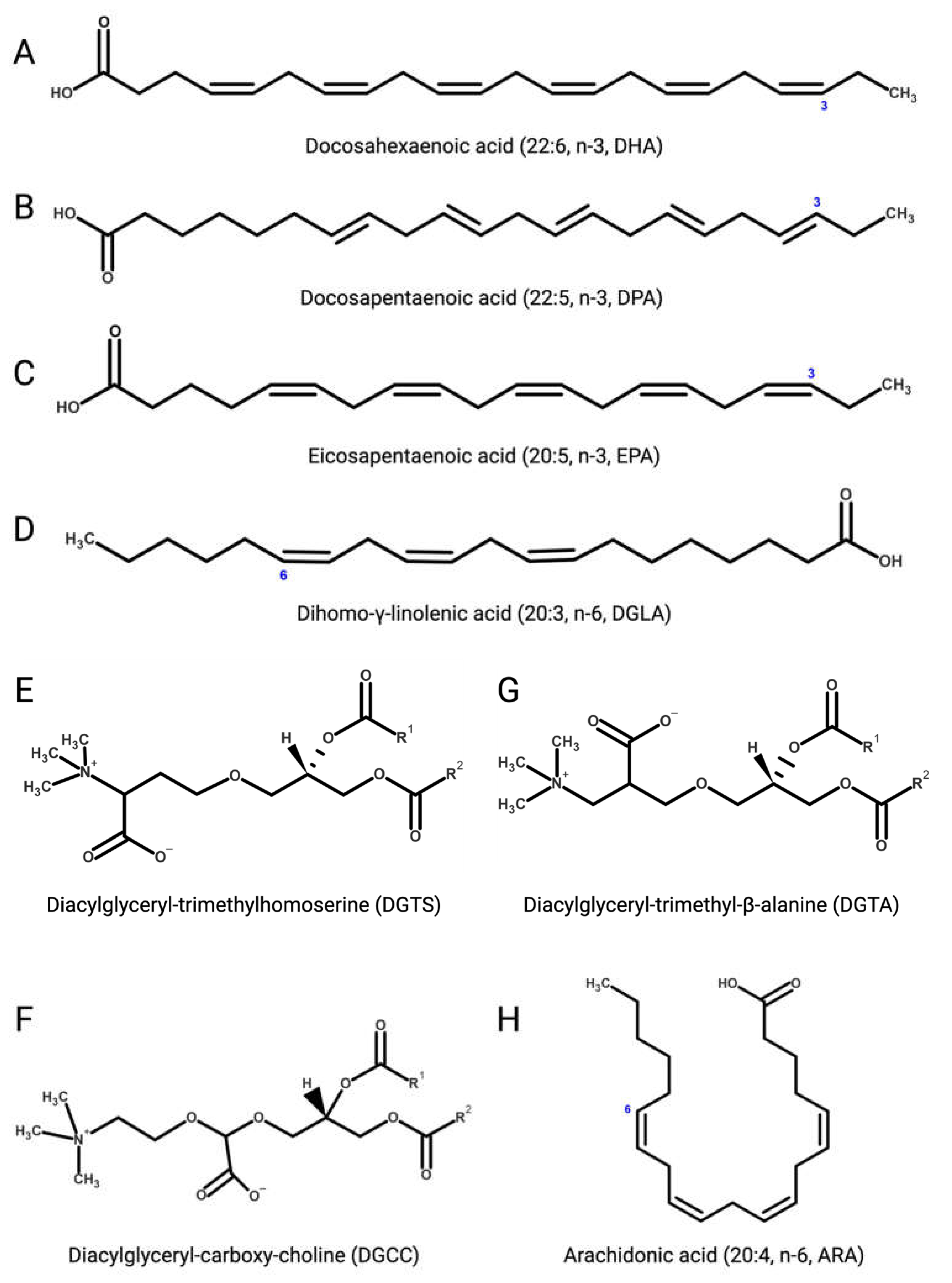
Figure 3. (A) Docosahexaenoic (DHA, 22:6, n-3); (B) docosapentaenoic (DPA, 22:5, n-3); (C) eicosapentaenoic (EPA, 20:5, n-3); (D) dihomo-γ-linolenic (DGLA, 20:3, n-6); (E) 1,2-diacylglyceryl-3-O-4’-(N,N,N-trimethyl)-homoserine (DGTS); (F) 1,2-diacylglyceryl-3-O-carboxy-(hydroxymethyl)-choline (DGCC); (G) 1,2-diacylglyceryl-3-O-2’-(hydroxymethyl)-(N,N,N-trimethyl)-β-alanine (DGTA); (H) arachidonic (ARA, 20:4, n-6).
2.3.2. Betaine Lipids
Betaine lipids are anti-inflammatory and immunomodulatory glycerolipids in which the phosphate and carbohydrate moieties attached to the glycerol backbone are replaced with positively charged ether-bond betaine groups. Betaine lipids are widely distributed in all clades of eukaryotic microalgae, where they act as acyl group donors upon membrane lipid remodelling and during the accumulation of storage neutral lipids [145][146]. Betaine lipids derive from the turnover of membrane phospholipids under abiotic stresses, mainly temperature and nutrient starvation [147][148][149]. The betaine lipid 1,2-diacylglyceryl-3-O-4’-(N,N,N-trimethyl)-homoserine (DGTS, Figure 3E) is the most abundant betaine lipid in microalgae, followed by 1,2-diacylglyceryl-3-O-carboxy-(hydroxymethyl)-choline (DGCC, Figure 3F), and 1,2-diacylglyceryl-3-O-2’-(hydroxymethyl)-(N,N,N-trimethyl)-β-alanine (DGTA, Figure 3G), with evidence of DGTS-mediated inhibition of the IKK-β kinase causing suppression of the NF-κB pathway and secretion of pro-inflammatory ILs by Th1 and Th2 cells, as recently reported with extracts from the oleaginous chlorophyte Chromochloris zofingiensis (Chlorophyceae) [150].References
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602.
- Meyer, A.; Cirpus, P.; Ott, C.; Schlecker, R.; Zähringer, U.; Heinz, E. Biosynthesis of Docosahexaenoic Acid in Euglena gracilis: Biochemical and Molecular Evidence for the Involvement of a Δ4-Fatty Acyl Group Desaturase. Biochemistry 2003, 42, 9779–9788.
- Kvien, T.K.; Uhlig, T.; Ødegård, S.; Heiberg, M.S. Epidemiological aspects of rheumatoid arthritis: The sex ratio. Ann. N. Y Acad. Sci. 2006, 1069, 212–222.
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905.
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397.
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatology 2020, 59, 2661–2670.
- Schäfer, C.; Keyßer, G. Lifestyle Factors and Their Influence on Rheumatoid Arthritis: A Narrative Review. J. Clin. Med. 2022, 11, 7179.
- Philippou, E.; Petersson, S.D.; Rodomar, C.; Nikiphorou, E. Rheumatoid arthritis and dietary interventions: Systematic review of clinical trials. Nutr. Rev. 2021, 79, 410–428.
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991.
- Cutolo, M.; Nikiphorou, E. Nutrition and Diet in Rheumatoid Arthritis. Nutrients 2022, 14, 888.
- Malavasi, V.; Soru, S.; Cao, G. Extremophile Microalgae: The potential for biotechnological application. J. Phycol. 2020, 56, 559–573.
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372.
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2021, 49, D1004–D1011.
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617.
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744.
- Barone, G.D.; Cernava, T.; Ullmann, J.; Liu, J.; Lio, E.; Germann, A.T.; Nakielski, A.; Russo, D.A.; Chavkin, T.; Knufmann, K.; et al. Recent developments in the production and utilization of photosynthetic microorganisms for food applications. Heliyon 2023, 9, e14708.
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545.
- Borowitzka, M.A. Chapter 9—Microalgae in Medicine and Human Health: A Historical Perspective. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 195–210.
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2.
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895.
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383.
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201.
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445.
- Singh, J.A.; Arayssi, T.; Duray, P.; Schumacher, H.R. Immunohistochemistry of normal human knee synovium: A quantitative study. Ann. Rheum. Dis. 2004, 63, 785–790.
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942.
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260.
- Makowski, L.; Chaib, M.; Rathmell, J.C. Immunometabolism: From basic mechanisms to translation. Immunol. Rev. 2020, 295, 5–14.
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219.
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2017, 8, 1958.
- Brown, K.D.; Claudio, E.; Siebenlist, U. The roles of the classical and alternative nuclear factor-κB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, 212.
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127.
- Schett, G.; Zwerina, J.; Firestein, G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 909–916.
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis—Immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429.
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2.
- Ni, S.; Shan, F.; Geng, J. Interleukin-10 family members: Biology and role in the bone and joint diseases. Int. Immunopharmacol. 2022, 108, 108881.
- Müller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; Laface, D.; Hutchins, B.; Laube, G.; et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385.
- Sandmann, G. Diversity and origin of carotenoid biosynthesis: Its history of coevolution towards plant photosynthesis. New Phytol. 2021, 232, 479–493.
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118.
- Gao, J.; Wang, H.; Yuan, Q.; Feng, Y. Structure and Function of the Photosystem Supercomplexes. Front. Plant Sci. 2018, 9, 357.
- Anjani, G.; Ayustaningwarno, F.; Eviana, R. Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J. Funct. Foods 2022, 99, 105303.
- Moia, V.M.; Leal Portilho, F.; Almeida Pádua, T.; Barbosa Corrêa, L.; Ricci-Junior, E.; Cruz Rosas, E.; Magalhaes Rebelo Alencar, L.; Savio Mendes Sinfronio, F.; Sampson, A.; Hussain Iram, S.; et al. Lycopene used as Anti-inflammatory Nanodrug for the Treatment of Rheumathoid Arthritis: Animal assay, Pharmacokinetics, ABC Transporter and Tissue Deposition. Colloids Surf. B Biointerfaces 2020, 188, 110814.
- Renju, G.L.; Muraleedhara Kurup, G.; Saritha Kumari, C.H. Anti-inflammatory activity of lycopene isolated from Chlorella marina on Type II Collagen induced arthritis in Sprague Dawley rats. Immunopharmacol. Immunotoxicol. 2013, 35, 282–291.
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Chapter Two—Assessing photoprotective functions of carotenoids in photosynthetic systems of plants and green algae. In Methods in Enzymology; Wurtzel, E.T., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 674, pp. 53–84.
- Zhao, K.; Zhou, T.; Yang, J.; Li, Y.; Qin, J.; Wang, S.; Li, D.; Chen, J.; Zheng, W.V. Lutein shows a protective effect against the aging of mesenchymal stem cells by downregulating inflammation. Int. Immunopharmacol. 2023, 116, 109749.
- Pick, U.; Zarka, A.; Boussiba, S.; Davidi, L. A hypothesis about the origin of carotenoid lipid droplets in the green algae Dunaliella and Haematococcus. Planta 2019, 249, 31–47.
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323.
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Dos Santos Nascimiento, L.B.; Tredici, M.R.; Luceri, C. A Comparative In Vitro Evaluation of the Anti-Inflammatory Effects of a Tisochrysis lutea Extract and Fucoxanthin. Mar. Drugs 2021, 19, 334.
- Sansone, C.; Pistelli, L.; Del Mondo, A.; Calabrone, L.; Fontana, A.; Noonan, D.M.; Albini, A.; Brunet, C. The Microalgal Diatoxanthin Inflects the Cytokine Storm in SARS-CoV-2 Stimulated ACE2 Overexpressing Lung Cells. Antioxidants 2022, 11, 1515.
- Satoh, A.; Tsuji, S.; Okada, Y.; Murakami, N.; Urami, M.; Nakagawa, K.; Ishikura, M.; Katagiri, M.; Koga, Y.; Shirasawa, T. Preliminary Clinical Evaluation of Toxicity and Efficacy of A New Astaxanthin-rich Haematococcus pluvialis Extract. J. Clin. Biochem. Nutr. 2009, 44, 280–284.
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993.
- Valenti, M.T.; Perduca, M.; Romanelli, M.G.; Mottes, M.; Dalle Carbonare, L. A potential role for astaxanthin in the treatment of bone diseases (Review). Mol. Med. Rep. 2020, 22, 1695–1701.
- Donoso, A.; González-Durán, J.; Muñoz, A.A.; González, P.A.; Agurto-Muñoz, C. Therapeutic uses of natural astaxanthin: An evidence-based review focused on human clinical trials. Pharmacol. Res. 2021, 166, 105479.
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 8161–8177.
- Zhang, L.; Chen, H.; Ding, K.; He, S.; Li, G.; Qu, J.; Qiao, Y.; Zhang, L.; Sui, X.; Fan, C.; et al. Astaxanthin intake alleviates gouty arthritis in patients and rats by modulating the levels of various inflammatory markers. J. Funct. Foods 2021, 87, 104823.
- Tamura, H.; Ishikita, H. Quenching of Singlet Oxygen by Carotenoids via Ultrafast Superexchange Dynamics. J. Phys. Chem. A 2020, 124, 5081–5088.
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17, 103.
- Zhang, J.; Sun, Z.; Sun, P.; Chen, T.; Chen, F. Microalgal carotenoids: Beneficial effects and potential in human health. Food Funct. 2014, 5, 413–425.
- Yang, Y.; Kim, B.; Lee, J.-Y. Astaxanthin Structure, Metabolism, and Health Benefits. 2014. Available online: https://www.jscimedcentral.com/public/assets/articles/nutrition-1-1003.pdf (accessed on 15 November 2023).
- Fukuzawa, K.; Inokami, Y.; Tokumura, A.; Terao, J.; Suzuki, A. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and α-tocopherol in liposomes. Lipids 1998, 33, 751–756.
- Bolin, A.P.; Macedo, R.C.; Marin, D.P.; Barros, M.P.; Otton, R. Astaxanthin prevents in vitro auto-oxidative injury in human lymphocytes. Cell Biol. Toxicol. 2010, 26, 457–467.
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457.
- Guerra, B.A.; Otton, R. Impact of the carotenoid astaxanthin on phagocytic capacity and ROS/RNS production of human neutrophils treated with free fatty acids and high glucose. Int. Immunopharmacol. 2011, 11, 2220–2226.
- Guerra, B.A.; Bolin, A.P.; Otton, R. Carbonyl stress and a combination of astaxanthin/vitamin C induce biochemical changes in human neutrophils. Toxicol Vitr. 2012, 26, 1181–1190.
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; Lutiis, M.A.d.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899.
- Kimble, L.; Mathison, B.; Chew, B.P. Astaxanthin mediates inflammatory biomarkers associated with arthritis in human chondrosarcoma cells induced with interleukin-1β. FASEB J. 2013, 27, 638.6.
- Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Yagi, M.; Yonei, Y. Inhibitory Effects of Astaxanthin on CML-HSA-Induced Inflammatory and RANKL-Induced Osteoclastogenic Gene Expression in RAW 264.7 Cells. Biomedicines 2022, 10, 54.
- Kumar, A.; Dhaliwal, N.; Dhaliwal, J.; Dharavath, R.N.; Chopra, K. Astaxanthin attenuates oxidative stress and inflammatory responses in complete Freund-adjuvant-induced arthritis in rats. Pharmacol. Rep. 2020, 72, 104–114.
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531.
- Wang, X.; Liu, Z.; Peng, P.; Gong, Z.; Huang, J.; Peng, H. Astaxanthin attenuates osteoarthritis progression via inhibiting ferroptosis and regulating mitochondrial function in chondrocytes. Chem.-Biol. Interact. 2022, 366, 110148.
- Budriesi, R.; Micucci, M.; Daglia, M.; Corazza, I.; Biotti, G.; Mattioli, L.B. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System. Biol. Life Sci. Forum 2022, 12, 31.
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13, 2857–2874.
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152.
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Cao, Y.; Xu, J.; Xue, C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497.
- Madhavi, D.; Kagan, D.; Seshadri, S. A Study on the Bioavailability of a Proprietary, Sustained-release Formulation of Astaxanthin. Integr. Med. 2018, 17, 38–42.
- Liu, X.; Xie, J.; Zhou, L.; Zhang, J.; Chen, Z.; Xiao, J.; Cao, Y.; Xiao, H. Recent advances in health benefits and bioavailability of dietary astaxanthin and its isomers. Food Chem. 2023, 404, 134605.
- Jafari, Z.; Bigham, A.; Sadeghi, S.; Dehdashti, S.M.; Rabiee, N.; Abedivash, A.; Bagherzadeh, M.; Nasseri, B.; Karimi-Maleh, H.; Sharifi, E.; et al. Nanotechnology-Abetted Astaxanthin Formulations in Multimodel Therapeutic and Biomedical Applications. J. Med. Chem. 2022, 65, 2–36.
- Li, B.; Lee, J.-Y.; Luo, Y. Health benefits of astaxanthin and its encapsulation for improving bioavailability: A review. J. Agric. Food Res. 2023, 14, 100685.
- Abdol Wahab, N.R.; Meor Mohd Affandi, M.M.R.; Fakurazi, S.; Alias, E.; Hassan, H. Nanocarrier System: State-of-the-Art in Oral Delivery of Astaxanthin. Antioxidants 2022, 11, 1676.
- Chen, S.; Wang, J.; Feng, J.; Xuan, R. Research progress of Astaxanthin nano-based drug delivery system: Applications, prospects and challenges? Front. Pharmacol. 2023, 14, 1102888.
- Hien, H.T.M.; Oanh, H.T.; Quynh, Q.T.; Thu, N.T.H.; Van Hanh, N.; Hong, D.D.; Hoang, M.H. Astaxanthin-loaded nanoparticles enhance its cell uptake, antioxidant and hypolipidemic activities in multiple cell lines. J. Drug Deliv. Sci. Technol. 2023, 80, 104133.
- Bai, S.-K.; Lee, S.-J.; Na, H.-J.; Ha, K.-S.; Han, J.-A.; Lee, H.; Kwon, Y.-G.; Chung, C.-K.; Kim, Y.-M. β-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-κB activation. Exp. Mol. Med. 2005, 37, 323–334.
- Lee, S.J.; Bai, S.K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-κB activation. Mol. Cells 2003, 16, 97–105.
- Priyadarshini, L.; Aggarwal, A. Astaxanthin inhibits cytokines production and inflammatory gene expression by suppressing IκB kinase-dependent nuclear factor κB activation in pre and postpartum Murrah buffaloes during different seasons. Vet. World 2018, 11, 782–788.
- Li, R.; Hong, P.; Zheng, X. β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2019, 90, 140–148.
- Lee, A.H.; Shin, H.-Y.; Park, J.-H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543.
- Pistelli, L.; Sansone, C.; Smerilli, A.; Festa, M.; Noonan, D.M.; Albini, A.; Brunet, C. MMP-9 and IL-1β as Targets for Diatoxanthin and Related Microalgal Pigments: Potential Chemopreventive and Photoprotective Agents. Mar. Drugs 2021, 19, 354.
- Vermeulen, L.; De Wilde, G.; Van Damme, P.; Vanden Berghe, W.; Haegeman, G. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003, 22, 1313–1324.
- Takeba, Y.; Suzuki, N.; Wakisaka, S.; Takeno, M.; Kaneko, A.; Asai, T.; Sakane, T. Involvement of cAMP responsive element binding protein (CREB) in the synovial cell hyperfunction in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 2000, 18, 47–55.
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419.
- Terazawa, S.; Nakajima, H.; Shingo, M.; Niwano, T.; Imokawa, G. Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion–independent manner. Exp. Dermatol. 2012, 21, 11–17.
- Widyaningrum, D.; Oktafika, R.A.; Cecilia, D. Microalgae pigments as a promising immunomodulating food ingredient: In silico study. IOP Conf. Ser. Earth Environ. Sci. 2022, 998, 012056.
- Pflug, K.M.; Sitcheran, R. Targeting NF-κB-Inducing Kinase (NIK) in Immunity, Inflammation, and Cancer. Int. J. Mol. Sci. 2020, 21, 8470.
- Zhao, L.; Tao, X.; Wan, C.; Dong, D.; Wang, C.; Xi, Q.; Liu, Y.; Song, T. Astaxanthin alleviates inflammatory pain by regulating the p38 mitogen-activated protein kinase and nuclear factor-erythroid factor 2-related factor/heme oxygenase-1 pathways in mice. Food Funct. 2021, 12, 12381–12394.
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544.
- Chen, B.; Ning, K.; Sun, M.-l.; Zhang, X.-A. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: A systematic review. Cell Commun. Signal. 2023, 21, 67.
- Tripathi, S.K.; Chen, Z.; Larjo, A.; Kanduri, K.; Nousiainen, K.; Äijo, T.; Ricaño-Ponce, I.; Hrdlickova, B.; Tuomela, S.; Laajala, E.; et al. Genome-wide Analysis of STAT3-Mediated Transcription during Early Human Th17 Cell Differentiation. Cell Rep. 2017, 19, 1888–1901.
- Maik-Rachline, G.; Zehorai, E.; Hanoch, T.; Blenis, J.; Seger, R. The nuclear translocation of the kinases p38 and JNK promotes inflammation-induced cancer. Sci. Signal 2018, 11, eaao3428.
- Yang, Y.; Kim, S.C.; Yu, T.; Yi, Y.-S.; Rhee, M.H.; Sung, G.-H.; Yoo, B.C.; Cho, J.Y. Functional Roles of p38 Mitogen-Activated Protein Kinase in Macrophage-Mediated Inflammatory Responses. Mediat. Inflamm. 2014, 2014, 352371.
- O’Neil, J.D.; Ammit, A.J.; Clark, A.R. MAPK p38 regulates inflammatory gene expression via tristetraprolin: Doing good by stealth. Int. J. Biochem. Cell Biol. 2018, 94, 6–9.
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448.
- Kim, E.A.; Kang, N.; Heo, S.Y.; Oh, J.Y.; Lee, S.H.; Cha, S.H.; Kim, W.K.; Heo, S.J. Antioxidant, Antiviral, and Anti-Inflammatory Activities of Lutein-Enriched Extract of Tetraselmis Species. Mar. Drugs 2023, 21, 369.
- Clayton, S.A.; MacDonald, L.; Kurowska-Stolarska, M.; Clark, A.R. Mitochondria as Key Players in the Pathogenesis and Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 673916.
- Kim, J.; Cha, Y.N.; Surh, Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23.
- Ferrándiz, M.L.; Nacher-Juan, J.; Alcaraz, M.J. Nrf2 as a therapeutic target for rheumatic diseases. Biochem. Pharmacol. 2018, 152, 338–346.
- Sigaux, J.; Bellicha, A.; Buscail, C.; Julia, C.; Flipo, R.M.; Cantagrel, A.; Laporte, F.; Beal, C.; Boissier, M.C.; Semerano, L. Serum Fatty Acid Profiles Are Associated with Disease Activity in Early Rheumatoid Arthritis: Results from the ESPOIR Cohort. Nutrients 2022, 14, 2947.
- Mustonen, A.-M.; Nieminen, P. Fatty Acids and Oxylipins in Osteoarthritis and Rheumatoid Arthritis—A Complex Field with Significant Potential for Future Treatments. Curr. Rheumatol. Rep. 2021, 23, 41.
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452.
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881.
- Demets, R.; Foubert, I. Chapter 1—Traditional and novel sources of long-chain omega-3 fatty acids. In Omega-3 Delivery Systems; García-Moreno, P.J., Jacobsen, C., Moltke Sørensen, A.-D., Yesiltas, B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–23.
- Fernandes, T.; Cordeiro, N. Microalgae as Sustainable Biofactories to Produce High-Value Lipids: Biodiversity, Exploitation, and Biotechnological Applications. Mar. Drugs 2021, 19, 573.
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113.
- Razali, W.A.W.; Pandhal, J. Outdoor pilot-scale cultivation and techno economic assessment of a novel omega-3 eicosapentaenoic acid over-producing Nannochloropsis oculata strain. Bioresour. Technol. Rep. 2023, 24, 10168.
- Brett, M.; Müller-Navarra, D. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw. Biol. 1997, 38, 483–499.
- Hixson, S.M.; Arts, M.T. Climate warming is predicted to reduce omega-3, long-chain, polyunsaturated fatty acid production in phytoplankton. Glob. Chang. Biol. 2016, 22, 2744–2755.
- Wang, T.; Tong, S.; Liu, N.; Li, F.; Wells, M.L.; Gao, K. The fatty acid content of plankton is changing in subtropical coastal waters as a result of OA: Results from a mesocosm study. Mar. Environ. Res. 2017, 132, 51–62.
- Puccinelli, E.; Sardenne, F.; Pecquerie, L.; Fawcett, S.E.; Machu, E.; Soudant, P. Omega-3 Pathways in Upwelling Systems: The Link to Nitrogen Supply. Front. Mar. Sci. 2021, 8, 664601.
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Progress. Lipid Res. 2019, 74, 31–68.
- Lopes, D.; Aveiro, S.S.; Conde, T.; Rey, F.; Couto, D.; Melo, T.; Moreira, A.S.P.; Domingues, M.R. Chapter 6—Algal lipids: Structural diversity, analysis and applications. In Functional Ingredients from Algae for Foods and Nutraceuticals, 2nd ed.; Dominguez, H., Pereira, L., Kraan, S., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 335–396.
- Kugler, A.; Zorin, B.; Didi-Cohen, S.; Sibiryak, M.; Gorelova, O.; Ismagulova, T.; Kokabi, K.; Kumari, P.; Lukyanov, A.; Boussiba, S.; et al. Long-Chain Polyunsaturated Fatty Acids in the Green Microalga Lobosphaera incisa Contribute to Tolerance to Abiotic Stresses. Plant Cell Physiol. 2019, 60, 1205–1223.
- Rousch, J.M.; Bingham, S.E.; Sommerfeld, M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003, 295, 145–156.
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825.
- Khozin-Goldberg, I.; Leu, S.; Boussiba, S. Microalgae as a Source for VLC-PUFA Production. Subcell. Biochem. 2016, 86, 471–510.
- Taipale, S.; Peltomaa, E.; Salmi, P. Variation in ω-3 and ω-6 Polyunsaturated Fatty Acids Produced by Different Phytoplankton Taxa at Early and Late Growth Phase. Biomolecules 2020, 10, 559.
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410.
- Lupette, J.; Benning, C. Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 2020, 178, 15–25.
- Nauroth, J.M.; Liu, Y.C.; Van Elswyk, M.; Bell, R.; Hall, E.B.; Chung, G.; Arterburn, L.M. Docosahexaenoic acid (DHA) and docosapentaenoic acid (DPAn-6) algal oils reduce inflammatory mediators in human peripheral mononuclear cells in vitro and paw edema in vivo. Lipids 2010, 45, 375–384.
- Novichkova, E.; Nayak, S.; Boussiba, S.; Gopas, J.; Zilberg, D.; Khozin-Goldberg, I. Dietary Application of the Microalga Lobosphaera incisa P127 Reduces Severity of Intestinal Inflammation, Modulates Gut-Associated Gene Expression, and Microbiome in the Zebrafish Model of IBD. Mol. Nutr. Food Res. 2023, 67, 2200253.
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424.
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Aiese Cigliano, R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Progress Lipid Res. 2019, 76, 101007.
- Ngoc Mai, D.T.; Ha, N.C.; Thom, L.T.; Hong, D.D. Initial studies on squalene from some marine microalgae isolated in Vietnam. Acad. J. Biol. 2013, 35, 333–341.
- Chen, X.; He, Y.; Ye, H.; Xie, Y.; Sen, B.; Jiao, N.; Wang, G. Different carbon and nitrogen sources regulated docosahexaenoic acid (DHA) production of Thraustochytriidae sp. PKU#SW8 through a fully functional polyunsaturated fatty acid (PUFA) synthase gene (pfaB). Bioresour. Technol. 2020, 318, 124273.
- Leyton, A.; Shene, C.; Chisti, Y.; Asenjo, J.A. Production of Carotenoids and Phospholipids by Thraustochytrium sp. in Batch and Repeated-Batch Culture. Mar. Drugs 2022, 20, 416.
- Jaritkhuan, S.; Suanjit, S. Species diversity and polyunsaturated fatty acid content of thraustochytrids from fallen mangrove leaves in Chon Buri province, Thailand. Agric. Nat. Resour. 2018, 52, 24–32.
- Dellero, Y.; Cagnac, O.; Rose, S.; Seddiki, K.; Cussac, M.; Morabito, C.; Lupette, J.; Aiese Cigliano, R.; Sanseverino, W.; Kuntz, M.; et al. Proposal of a new thraustochytrid genus Hondaea gen. nov. and comparison of its lipid dynamics with the closely related pseudo-cryptic genus Aurantiochytrium. Algal Res. 2018, 35, 125–141.
- Olsen, P.M.; Kósa, G.; Klüver, M.; Kohler, A.; Shapaval, V.; Horn, S.J. Production of docosahexaenoic acid from spruce sugars using Aurantiochytrium limacinum. Bioresour. Technol. 2023, 376, 128827.
- Aini, U.N.; Lunprom, S.; Reungsang, A.; Salakkam, A. Docosahexaenoic acid (DHA) production by Aurantiochytrium limacinum using cassava pulp hydrolysate as an alternative low-cost carbon source. Front. Mar. Sci. 2022, 9, 985119.
- Didrihsone, E.; Dubencovs, K.; Grube, M.; Shvirksts, K.; Suleiko, A.; Suleiko, A.; Vanags, J. Crypthecodinium cohnii Growth and Omega Fatty Acid Production in Mediums Supplemented with Extract from Recycled Biomass. Mar. Drugs 2022, 20, 68.
- Ding, J.; Fu, Z.; Zhu, Y.; He, J.; Ma, L.; Bu, D. Enhancing docosahexaenoic acid production of Schizochytrium sp. by optimizing fermentation using central composite design. BMC Biotechnol. 2022, 22, 39.
- Patel, A.; Liefeldt, S.; Rova, U.; Christakopoulos, P.; Matsakas, L. Co-production of DHA and squalene by thraustochytrid from forest biomass. Sci. Rep. 2020, 10, 1992.
- Leong, H.Y.; Su, C.-A.; Lee, B.-S.; Lan, J.C.-W.; Law, C.L.; Chang, J.-S.; Show, P.L. Development of Aurantiochytrium limacinum SR21 cultivation using salt-rich waste feedstock for docosahexaenoic acid production and application of natural colourant in food product. Bioresour. Technol. 2019, 271, 30–36.
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255.
- Allen, K.M.; Habte-Tsion, H.-M.; Thompson, K.R.; Filer, K.; Tidwell, J.H.; Kumar, V. Freshwater microalgae (Schizochytrium sp.) as a substitute to fish oil for shrimp feed. Sci. Rep. 2019, 9, 6178.
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of oil from Schizochytrium sp. (strain ATCC 20889) for use in infant and follow-on formula as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07083.
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504.
- Cañavate, J.P.; Armada, I.; Ríos, J.L.; Hachero-Cruzado, I. Exploring occurrence and molecular diversity of betaine lipids across taxonomy of marine microalgae. Phytochemistry 2016, 124, 68–78.
- Hoffmann, D.Y.; Shachar-Hill, Y. Do betaine lipids replace phosphatidylcholine as fatty acid editing hubs in microalgae? Front. Plant Sci. 2023, 14, 1077347.
- Martin, P.; Van Mooy, B.A.S.; Heithoff, A.; Dyhrman, S.T. Phosphorus supply drives rapid turnover of membrane phospholipids in the diatom Thalassiosira pseudonana. ISME J. 2011, 5, 1057–1060.
- Murakami, H.; Nobusawa, T.; Hori, K.; Shimojima, M.; Ohta, H. Betaine Lipid Is Crucial for Adapting to Low Temperature and Phosphate Deficiency in Nannochloropsis. Plant Physiol. 2018, 177, 181–193.
- Oishi, Y.; Otaki, R.; Iijima, Y.; Kumagai, E.; Aoki, M.; Tsuzuki, M.; Fujiwara, S.; Sato, N. Diacylglyceryl-N,N,N-trimethylhomoserine-dependent lipid remodeling in a green alga, Chlorella kessleri. Commun. Biol. 2022, 5, 19.
- Leitner, P.D.; Jakschitz, T.; Gstir, R.; Stuppner, S.; Perkams, S.; Kruus, M.; Trockenbacher, A.; Griesbeck, C.; Bonn, G.K.; Huber, L.A.; et al. Anti-Inflammatory Extract from Soil Algae Chromochloris zofingiensis Targeting TNFR/NF-κB Signaling at Different Levels. Cells 2022, 11, 1407.
More
