Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Edoardo Cutolo and Version 3 by Rita Xu.
Rheumatoid arthritis (RA) is an invalidating chronic autoimmune disorder characterized by joint inflammation and progressive bone damage. Dietary intervention is an important component in the treatment of RA to mitigate oxidative stress, a major pathogenic driver of the disease. Alongside traditional sources of antioxidants, microalgae—a diverse group of photosynthetic prokaryotes and eukaryotes—are emerging as anti-inflammatory and immunomodulatory food supplements.
- photosynthesis
- polyunsaturated fatty acids
- carotenoids
- oxylipins
- xanthophylls
- antioxidants
- functional foods
- synthetic biology
1. Introduction
Chronic inflammation is a defining feature of autoimmune diseases, a group of conditions in which immunological self-tolerance is disturbed due to the recognition of autoantigens by immune cells. Rheumatoid arthritis (RA), the most common chronic inflammatory arthropathy [1][2][1,2], is a systemic autoimmune disorder affecting the synovial joints, with a higher incidence in women [3]. RA displays a complex pathophysiology involving the upregulation of pro-inflammatory mediators (interleukins, ILs) and enhanced production of reactive oxygen species (ROS) [4][5][4,5]. Both genetic and modifiable lifestyle factors contribute to the risk of RA predisposition [6][7][6,7], with diet highly influencing disease activity [8][9][8,9]. In particular, a high antioxidant intake is known to reduce onset risk and to ameliorate the clinical course of the disease [10], therefore, the identification of new sources of antioxidant and anti-inflammatory molecules is of high clinical relevance.
Microalgae are photosynthetic prokaryotes and eukaryotes adapted to diverse environments, including extreme habitats [11][12][11,12], which are consumed in human nutrition as sources of proteins and other bioactive compounds [13][14][15][16][17][13,14,15,16,17]. Several species are non-toxic producers of essential vitamins, lipids, and pigments of therapeutic value [18][19][20][21][22][18,19,20,21,22], which could be employed as complementary agents in the management of chronic inflammatory diseases. Moreover, the fast life cycle and light-powered autotrophic metabolism of microalgae allows for large-scale cultivation with lower inputs compared with heterotrophic microorganisms [23].
Pathogenesis and Mediators of Rheumatoid Arthritis
Although the exact etiology of RA remains unknown, the balance between immune cells and the production of inflammatory ILs in the connective tissue that lines the joint capsule (synovium) is altered in the disease onset and progression [4][5][4,5]. The healthy synovium consists of a thin lining layer of fibroblasts covering a connective tissue surrounded by blood vessels and enriched in fibroblasts, and innate and adaptive immune cells: the sub-lining layer [24] (Figure 1A). In RA, the lining layer is hyperplastic while the sub-lining layer is infiltrated with B-cells, monocyte-derived macrophages, autoantibody-secreting plasma cells, and differentiated cytotoxic CD4+ T-cells involved in the breakdown of tissue tolerance [25][26][27][25,26,27]. The release of pro-inflammatory ILs by monocyte-derived M1 macrophages, and osteoclast activation cause progressive bone resorption [28], and autoantibodies produced by differentiated plasma cells further contribute to joint damage [29].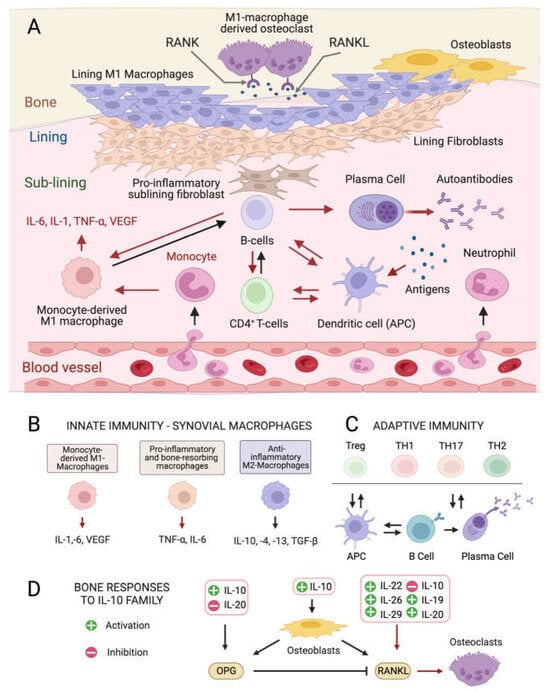
Figure 1. Cellular composition of the synovial membrane, its interaction with bone tissue and immune cell types, and related mediators involved in inflamed RA joints. (A) In healthy conditions, the synovium consist of a thin lining layer of lining fibroblasts in association with lining M1 directly exposed to the bone tissue. The underlying sub-lining layer is a connective tissue enriched in blood vessels, adipocytes, fibroblasts, and both innate and adaptive immune cells. The inflamed RA synovium is characterized by a hyperplastic lining layer surrounded by proinflammatory sub-lining fibroblasts and a massive infiltration of B-cells, monocyte-derived macrophages, autoantibody-secreting plasma cells, and differentiated cytotoxic effector memory CD4+ T-cells in the sub-lining layer. The secretion of pro-inflammatory interleukins (IL-1: interleukin 1; IL-6: interleukin 6; TNF- α: tumor necrosis factor-alpha) by activated immune cells stimulates the production of the soluble cytokine Mediator Receptor Activator of Nuclear Factor Kappa-Β Ligand (RANKL), which binds to its receptor RANK on monocytes and macrophages causing their differentiation into bone-resorbing osteoclasts. Red arrows indicate pro-inflammatory processes. (B) Interleukin (IL) isoforms produced by different types of synovial innate immune cells and macrophages (IL-6: interleukin 6; IL-1: interleukin 1; TNF-α: tumor necrosis factor-alpha; TNF-β: tumor necrosis factor-beta; VEGF: vascular endothelial growth factor). (C) Differentiation and interconversion of adaptive immune cells (Treg: regulatory T cells; TH1: T helper 1 cells; TH 17: T helper 17 cells; TH2: T helper 2 cells; B cells; APCs: antigen-presenting cells). (D) Effects of secreted ILs on bone-remodeling processes depending on the osteoprotegerin (OPG)–RANKL axis, which regulates the differentiation of osteoclasts in bone-resorbing osteoclasts.
2. Anti-Inflammatory and Immunomodulatory Metabolites from Microalgae
2.1. Carotenes and Xanthophylls
Like bacteria, fungi, and plants, microalgae synthetize C40 lipophilic pigments consisting of a polyene chain of conjugated double bonds (Figure 2) with terminally linked ionone rings known as carotenoids [37][38][37,38]. β-carotene (Figure 2B)—a structural element of photosystems [39]—and the antioxidant lycopene (Figure 2A) are anti-inflammatory carotenes [40][41][40,41] usually introduced in the diet with carrots (Daucus carota) and tomatoes (Solanum lycopersicum), respectively, although present also in microalgae [42]. Xanthophylls are oxygenated carotenoids containing hydroxyl and ketone groups in the ionone rings, which serve different functions in phototrophs. The non-ketolated xanthophyll lutein (Figure 2C) participates in light-harvesting and photoprotection, while the ketocarotenoid astaxanthin (ASTX, Figure 2D) scavenges harmful ROS generated by photosynthetic electron transport under excess light [43]. Lutein is an anti-inflammatory carotenoid [44] abundantly found in green leafy vegetables and egg yolk, while ASTX is a potent antioxidant uniquely synthetized by a few microalgal species. Abiotic stresses induce a hypercarotenogenic response in several chlorophytes, including the halophile Dunaliella salina (Chlorophyceae), which overaccumulates β-carotene in lipid bodies (plastoglobules) inside the chloroplast [45], and in the freshwater species Haematococcus pluvialis (Haematococcaceae), which forms haematocysts filled with ASTX-rich cytoplasmic lipid droplets [46]. Other microalgal xanthophylls with anti-inflammatory and immunomodulatory properties are fucoxanthin and diatoxanthin produced by several diatoms (stramenopiles) and by the haptophyte Tisochrysis lutea (Coccolithophyceae) (Figure 2E,F) [47][48][47,48]. As discussed in the following paragraphs, carotenoids appear to interfere in all major pro-inflammatory pathways implicated in the onset and progression of RA.Astaxanthin: The Red Gold of Algae
With recognized safety for human consumption [49], approved Novel Food status [50], and an established role in promoting bone homeostasis in degenerative skeletal diseases [51], ASTX is the microalgal pigment of highest biopharmaceutical value. Clinical studies have shown that ASTX intake reduces the levels of systemic inflammatory biomarkers [52][53][52,53] and potentiates the pain-relieving effect of anti-inflammatory therapies [54]. The pharmacological effects of ASTX derive from its strong antioxidant-activity mediated via ROS quenching [55] (Figure 2G, top panel) and direct free radical scavenging [56][57][56,57] (Figure 2G, bottom panel). This amphipathic molecule is symmetrically arranged within the lipid bilayer [58], thus exerting antioxidant activity on both intra- and extracellular environments. Notably, the higher number of hydroxyl groups compared with other carotenoids confers to ASTX superior ROS-detoxifying capacity [59]. Early studies showed that ASTX suppressed ROS production [60][61][62][63][60,61,62,63] and secretion of pro-inflammatory ILs by cultured human-activated monocytes [64]. Moreover ASTX stimulated the expression of ROS-scavenging enzymes in chondrocytes challenged with IL-1β [65], and inhibited pro-inflammatory and osteoclastogenic gene expression in macrophages challenged with RANKL [66]. Lastly, the administration of ASTX promoted cartilage health in animal models of arthritis and osteoasthritis [67][68][69][67,68,69].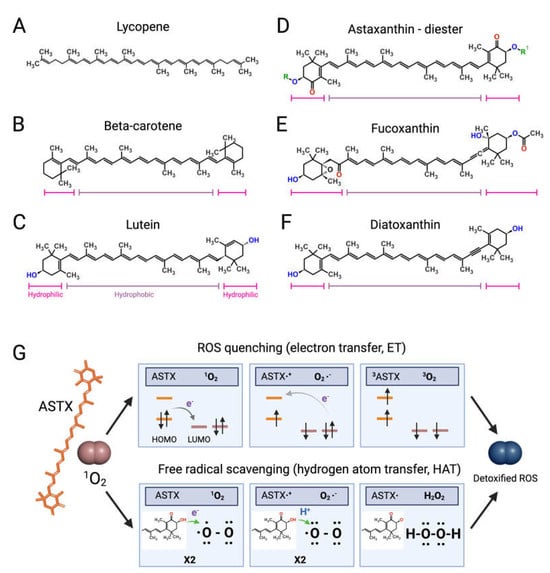
Figure 2. Structures of microalgal carotenoids and ROS detoxification mechanisms of astaxanthin. Lycopene (A), beta-carotene (B), lutein (C), astaxanthin (ASTX, (D)), fucoxanthin (E), and diatoxanthin (F). Pink and purple bars indicate the hydrophobic and hydrophilic regions of the molecules, respectively; in red, the oxygen of the keto groups, and in blue, the oxygen of the carboxylic groups; in green, the R and R’ functional groups of astaxanthin. Panel (G) outlines the two main routes of ASTX-mediated singlet molecule oxygen (1O2) detoxification. The top pathway is based on an electron transfer process involving: (i) the formation of a weakly bound ASTX-1O2 complex followed by direct electron transfer from the highest occupied molecular orbital (HOMO) of ASTX to the lowest unoccupied molecular orbital (LUMO) of singlet oxygen (1O2), and the formation of radicals; (ii) a reverse reaction restoring the electron distribution between the two molecules. The overall process converts 1O2 to its triplet unreactive form (3O2) upon spin inversion, while ASTX is restored from 3ASTX via internal conversion. The bottom pathway shows the free radical scavenging activity based on a two-step transfer involving both an electron and proton (H+) from ASTX to 1O2. The formed hydrogen peroxide is readily removed by peroxidase enzymes while ASTX is spontaneously restored by ascorbate. These mechanisms of action are iterative, meaning that a single ASTX molecule can perform multiple ROS detoxification cycles.
2.2. Anti-Inflammatory Mechanisms of Action of Astaxanthin and Other Carotenoids
2.2.1. NF-κB Pathway
ASTX and β-carotene interfere with the NF-κB pathway blocking the translocation of the NF-κB transcription factor to the nucleus, thereby suppressing ROS and pro-inflammatory gene expression. This effect is likely mediated through targeting the Inhibitor of the NF-κB γ subunit (IKK-γ) of the IkB kinase complex [81][82][83][84][81,82,83,84]. This prevents the phosphorylation and subsequent proteasome-mediated degradation of the IkBα binding factor, which abolishes the release of NF-κB [30]. A similar inhibitory effect has been proposed for fucoxanthin and diatoxanthin [85][86][85,86]. The Mitogen- and Stress-activated protein Kinase-1 (MSK1) is a nucleus-localized factor, which activates the NF-κB pathway [87] and the transcriptional regulator cAMP-responsive Element-Binding Protein (CREB) [88]. Phosphorylated CREB binds CREB-Responsive Elements (CRE) promoting pro-inflammatory gene expression [89]. These events are suppressed by ASTX, which inhibits MSK1 autophosphorylation [90]. Lastly, in silico simulations suggested that ASTX and β-carotene extracellularly interact with IL-6 and TNF-α, preventing their binding to membrane receptors [91]. ASTX may also interact with the NF-κB-Inducing Kinase (NIK) and block the phosphorylation of the IKK-α subunit of the IkB α kinase complex, suppressing the NF-κB pathway [92].2.2.2. JAK2/STAT3 and JNK/p38 MAPK Pathways
β-carotene and ASTX further modulate the pro-inflammatory pathways mediated by the JNK/p38 MAPK [93] and JAK2/STAT3 kinases [84][94][84,94], the latter responding to IL-6 in the pathogenesis of RA and osteoarthritis [31][95][31,95]. Phosphorylation of STAT3 dimers by JAK2 induces nuclear translocation and the differentiation of CD4+ T cells into the highly reactive T helper 17 (Th17) cell type [96]. TNFs and IL-1 activate the JNK/p38 MAPK pathway starting a phosphorylation cascade ending with JNK/p38 MAPK nuclear translocation [97], and phosphorylation of pro-inflammatory transcription factors (ELK1, MEF2, ATF2, and STAT1) [98] and of the MAPK-activated kinase 2 (MK2), which, in turn, targets the tristetrapolin (TTP) factor, promoting stabilization of IL mRNAs [99]. These oxidant-sensitive inflammatory pathways are also modulated by lutein [100], as reported using extracts enriched in this xanthophyll from different species of the chlorophyte genus Tetraselmis (Chlorodendrophyceae) [101].2.2.3. Other Pro-Inflammatory Pathways Targeted by Microalgal Carotenoids
Mitochondrial disfunction is a key pathogenic driver in RA [102], and ASTX was reported to attenuate organellar ROS production in human chondrocytes treated with IL-1β [69]. In addition to suppressing pro-inflammatory pathways, ASTX is also suggested to promote cartilage homeostasis via the transcriptional regulator nuclear factor-erythroid 2-related factor 2 (Nrf2) [68][93][68,93]. ASTX is suggested to stabilize and promote the nuclear translocation of Nfr2, which binds so-called antioxidant response elements (AREs), enhancing the expression of anti-inflammatory and ROS-detoxifying genes [103][104][103,104].2.3. Lipids and Their Derivatives
A hallmark of RA is the altered fatty acid profile of the synovium [105][106][105,106], while the intake of polyunsaturated fatty acids (PUFAs) correlates with joint health and mitigates the risk of RA onset [107]. Microalgal mass cultivation is a more sustainable way to derive functional lipids compared with cold water fish [108][109][110][111][112][108,109,110,111,112]. Phytoplankton occupies the lowest trophic level in oceans and freshwater basins, representing the primary PUFAs producer in aquatic food webs [113]. Global warning and ocean acidification are predicted to affect phytoplankton ecology [114][115][114,115], thus reducing PUFAs availability to higher trophic levels and, eventually, putting at risk the supply for human nutrition [116]. Moreover, upon stress acclimation, microalgae synthetise a wider range of anti-inflammatory and immunomodulatory lipids compared to animals [117][118][119][120][121][117,118,119,120,121].2.3.1. Long-Chain Polyunsaturated Fatty Acids
Several microalgae accumulate very long-chain PUFAs [122][123][122,123], including the omega-3 (ω-3, n-3) PUFAs [124] α-linolenic (18:3), docosahexaenoic (DHA, 22:6, n-3, Figure 3A), docosapentaenoic (DPA, n-3, 22:5, Figure 3B), and eicosapentaenoic (EPA, 20:5, n-3, Figure 3C) acids but also ω-6 PUFAs like arachidonic (ARA, 20:4, n-6, Figure 3H), γ-linolenic (18:3), linoleic (18:2), and dihomo-γ-linolenic (DGLA, 20:3, n-6, Figure 3D) acids. These molecules are biosynthetic precursors of anti-inflammatory signaling molecules and interfere with pro-inflammatory pathways [125][126][125,126].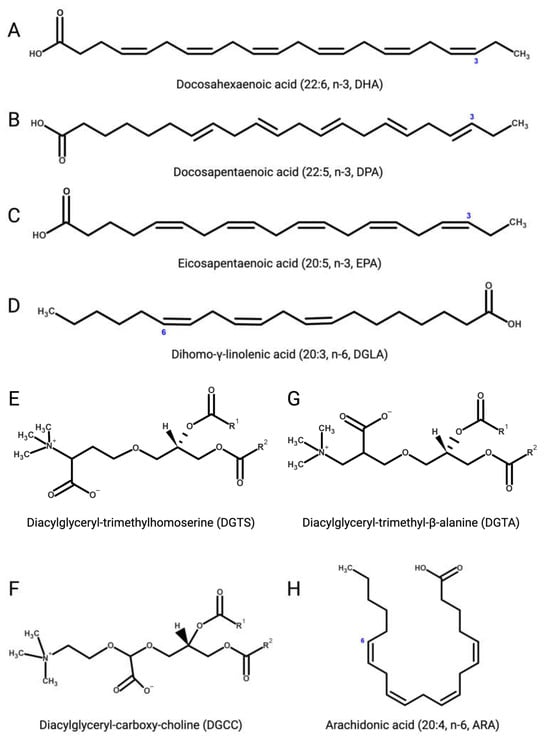
Figure 3. (A) Docosahexaenoic (DHA, 22:6, n-3); (B) docosapentaenoic (DPA, 22:5, n-3); (C) eicosapentaenoic (EPA, 20:5, n-3); (D) dihomo-γ-linolenic (DGLA, 20:3, n-6); (E) 1,2-diacylglyceryl-3-O-4’-(N,N,N-trimethyl)-homoserine (DGTS); (F) 1,2-diacylglyceryl-3-O-carboxy-(hydroxymethyl)-choline (DGCC); (G) 1,2-diacylglyceryl-3-O-2’-(hydroxymethyl)-(N,N,N-trimethyl)-β-alanine (DGTA); (H) arachidonic (ARA, 20:4, n-6).
