Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lingjun Li and Version 2 by Jason Zhu.
Owing to high efficacy and safety, natural medicines have found their way into the field of cancer therapy over the past few decades. However, the effective ingredients of natural medicines have shortcomings of poor solubility and low bioavailability. Nanoparticles can not only solve the problems above but also have outstanding targeting ability. Targeting preparations can be classified into three levels, which are target tissues, cells, and organelles. On the premise of clarifying the therapeutic purpose of drugs, one or more targeting methods can be selected to achieve more accurate drug delivery and consequently to improve the anti-tumor effects of drugs and reduce toxicity and side effects.
- nanoparticles
- tumor targeting
- natural medicines
1. EPR Effect-Mediated Drug Targeting
Owing to the high permeability of tumor blood vessels, nano-preparation with particles of a size less than 200 nm can enter the tumor stroma and be retained by impaired the lymphatic system. This phenomenon is known as the high permeability and retention effect of solid tumors (EPR effect) [1][22]. It is generally believed that the delivery system mediated by the EPR effect can effectively deliver nano-carriers to tumor tissues through passive transport [2][3][23,24]. In recent years, a series of studies on nano-preparation have been carried out at home and abroad, including micelle, liposome, nanoemulsion, and other dosage forms.
1.1. Micelle
When surfactant concentrations exceed the critical micelle concentrations (CMCs), soluble surfactants start to attract and associate with each other together to form micelles in an aqueous solution [4][25]. It has been repeatedly shown that the encapsulation of hydrophobic bioactive compounds inside this carrier system can improve their water solubility and bioavailability. According to the molecular weight of surfactants, micelles can be divided into low molecular micelles and polymer micelles.
As traditional excipients in the pharmaceutical field, low molecular surfactants are also good candidates for the preparation of micelles. In the early stage, phospholipids (amphoteric surfactants) and bile salts (anionic surfactants) were commonly used to prepare micelles, which could increase the solubility and anti-tumor efficacy of drugs [5][6][7][26,27,28]. Taking bile salts and phosphatidylcholine as carrier materials, Jiao et al. [5][26] prepared ISA (andrographolide derivative)-loaded mixed micelles with an encapsulation efficiency of 86.34%, a drug-loading rate of 4.87%, and an average particle size of 148.3 nm. The pharmacokinetic experiment displayed that compared with free drugs, the area under the curve (AUC0–12 h), in vivo retention time (MRT), and elimination half-life (t1/2) of micelles had increased by 2.62, 1.47, and 1.40 times, respectively, indicating that micelles could effectively improve the blood circulation time and bioavailability of ISA.
Despite all of these advantages, the application of micelles prepared from bile salts is frequently hampered because the alkaline micellar system is unsuitable for drugs that are unstable in alkaline environments. Non-ionic surfactants, which have been applied in establishing drug-carrying micelles, are a promising alternative carrier material for bile salts and can overcome the aforementioned drawback. Liang et al. [8][29] designed a paclitaxel (PTX)-loaded phospholipid-Tween-80 mixed micelle that exhibited stronger cytotoxic activity to cervical cancer cells HeLa and lung cancer cells A549 than free PTX (p < 0.01). The mechanism of action might be that Tween-80 could disrupt fatty molecules and bilayer membranes, evidently enhancing the permeability of cell membranes to PTX. An in vivo pharmacokinetic experiment demonstrated that the mixed micelle possessed higher bioavailability, with AUC0–t increasing by 1.3 times compared to the free PTX.
However, low molecular micelles have many inadequacies, including inflexibility in design and restricted cytotoxic effects on cancer cells and, which are more important, low molecular surfactants such as Tween-80 are the main anaphylactoid constituents of natural medicine injections. These factors have limited their clinical applications [9][10][30,31]. Therefore, in recent years, researchers have mostly switched to using amphiphilic polymers with low toxicities, excellent biodegradability, and satisfactory biocompatibility as carrier materials for the preparation of drug-carrying micelles.
Polymeric micelles, a disperse system with core–shell structure, are formed by a self-assembly of amphiphilic block copolymers in an aqueous solution. The average particle size of polymeric micelles ranges from 20 to 200 nm [11][12][32,33]. Amphiphilic copolymer carrier materials, including diblock, triblock, or pentablock copolymers (AB, ABA, ABC, or ABCBA block copolymers) [11][13][14][15][32,34,35,36], are commonly synthesized from two or more hydrophilic and hydrophobic copolymers through the esterification reaction, ring opening polymerization, or other methods [16][17][13,37]. The normally used raw materials can be divided into three categories: (1) hydrophilic polymers, such as polyethylene glycol (PEG), poly (vinyl pyrrolidone), poly(2-vinylpyridine), etc. [15][18][19][36,38,39]; (2) hydrophobic polymers, such as poly lactic-co-glycolic acid (PLGA), polycaprolactone (PCL), polylactic acid (PLA), etc. [15][20][21][22][36,40,41,42]; (3) amphiphilic block copolymers, mainly including D-alpha-tocopheryl polyethylene glycol succinate (TPGS), Soluplus®, and Pluronic® (F127, F68, and P123) [16][23][24][25][26][27][28][29][13,43,44,45,46,47,48,49]. During the micellization process, hydrophobic chain segments aggregate internally to form an inner core, serving as a reservoir for poorly water-soluble drugs. The hydrophilic outer corona is mainly composed of hydrophilic segments that can refrain from the clearance effect of the endothelial network system and prevent micellar particles from aggregating [4][25]. Such a core–shell structure not only enables the polymer to be well dispersed in aqueous solution but also provides a sufficient hydrophobic microenvironment for insoluble drugs due to its large relative molecular weight [25][45]. Therefore, compared with low molecular micelles, the encapsulation efficiency, drug loading, stability, anti-tumor effect, and bioavailability of polymer micelles are significantly improved [30][50]. Andrographolide (ADG) isolated from Andrographis paniculata (Burm. f.) Nees has anti-cancer and anti-inflammatory activities, but high hydrophobicity and poor bioavailability limit its clinical application [31][51]. Therefore, researchers prepared PLGA-PEG-PLGA/ADG polymer micelles with encapsulation efficiency and drug loading of 92% and 8.4%, respectively. The particle size was 124.3 ± 6.4 nm and could remain stable even after 15 days stored at 4 °C. ADG-loaded micelles had more outstanding anti-tumor ability and higher bioavailability compared to free ADG. In vitro tests proved that after 48 h of treatment, ADG micelles induced stronger cytotoxicity on breast cancer cell lines MAD-MB-231 than free drugs, with IC50 values of 7.45 ± 1.21 and 19.4 ± 2.52 µM, respectively. This might be related to the effect of inhibiting G2/M phase cell cycle and promoting cell apoptosis. In vivo experiments demonstrated that ADG micelles could continuously release within 48 h, and AUC0–∞ and MRT increased by 2.7- and 2.5-fold, respectively, compared to the original drug [32][52].
Polymer-mixed micelles (PMMs), formed by two or more different types of copolymers, can eliminate complex synthesis schemes of carrier materials, improve the stability of nano-micelles, enhance the compatibilization of hydrophobic compounds, and increase the anti-tumor efficacy of drugs [27][33][47,55]. In addition, PMMs have a smaller particle size (usually less than 100 nm), which is more conducive to cellular uptake and is a promising drug delivery system [33][34][55,56].
It is widely acknowledged that TPGS, which possesses inhibitory effects against P-glycoprotein (P-gp) overexpressing in multidrug-resistant cancer cells, has been a well-behaved carrier material for antineoplastic agents [35][36][37][54,57,58]. Nanoparticles utilizing TPGS individually to encapsulate CUR were regarded as an effective and safe delivery platform for oral administration, which were able to avoid degradation of the drug in the gastrointestinal tract and could be used for the treatment of colorectal cancer [36][57]. Despite its beneficial potential, the utilization of TPGS was still restricted due to high CMC and poor anti-dilution ability, making it difficult to maintain stability in blood circulation when administered intravenously. In view of these properties, F127 and P123 were added into the micellar system to prepare a CUR-loaded polymer-mixed micelle (CUR@NPT100), which held promise for the treatment of cervical cancer [35][54]. On the one hand, from the properties of the polymer carrier materials, the CMC values of the mixed copolymer and simplex TPGS were approximately 0.02 and 0.2 mg/mL, respectively, indicating that the copolymer micelles were expected to have great stability after dilution in the blood stream. On the other hand, from the perspective of efficacy, compared with non-cancerous cells NIH3T3, the mixed micelle significantly promoted the selective uptake of CUR by cervical cancer cells HeLa. Therefore, at the same drug concentration (2 μg/mL) for 48 h, CUR@NPT100 did not show evident cytotoxicity to NIH3T3 cells (the cell viability was approximately 85%) and showed a strong inhibitory effect on HeLa cells (the cell viability was about 55%). Moreover, the addition of TPGS to polymeric micelles can also significantly improve the encapsulation efficiency and bioavailability of drugs. When Soluplus/TPGS (3:2) were used as the carrier materials instead of Soluplus alone, the encapsulation efficiency of diosgenin increased from 66.7% to 92.6%, and the drug-loading rate increased from 3.3% to 4.6% [27][47]. When appropriate amounts of TPGS were added into HA-SS-PLA/PTX micelles, the t1/2, MRT, AUC0–∞, and peak concentration (Cmax) were raised by a factor of 1.33, 1.53, 2.05, and 1.33, respectively, indicating that mixed micelles could increase the retention time of simplex polymer micelles in vivo and improve the bioavailability of drugs [38][59]. Polymer micelles have attractive flexibility in design and can be composed of copolymers with multifarious physical and chemical properties, which is perfectly suitable for drugs with different degrees of hydrophobicity. Currently applied in establishing drug delivery system, polymer micelles have more practical meanings and can be considered as an ideal drug administration strategy against cancer.
1.2. Liposome
Liposomes, which can encapsulate or incorporate drugs into lipid bilayers, have many superiorities, such as sustained release, low toxicity, high stability, and strong permeability [39][40][41][60,61,62]. Once in the bloodstream, conventional liposomes would be coated with a series of plasma proteins, such as immunoglobulins and complements, giving rise to enhanced affinity with mononuclear macrophages, which would make it easy to be cleared in systemic circulation and prevent it from exerting long-lasting effects [42][43][63,64]. Therefore, researchers have been searching for more suitable carrier materials with the aim to obtain long-circulating function.
On the one hand, liposomes can achieve long-term circulation function through biological modifications. Erythrocytes, a type of circulating cell, has great biocompatibility, biodegradability, and long circulation properties. An increasing number of studies have confirmed that nanocarriers coated with erythrocyte membranes had preponderances in terms of long circulation and biocompatibility [44][45][46][65,66,67]. Zhong et al. [47][68] designed a novel biomimetic liposome coated with erythrocyte membranes and a co-loading of triptolide and celastrol (C + T/RBCm@Lip), which could effectively evade recognition and clearance by macrophages. Erythrocyte membrane coating could not only avoid the rapid clearance of an immune system and prolong the blood circulation of liposomes but also increase the uptake of liposomes by tumor cells and enhance the inhibitory effects of the drug on the growth of HepG2 cells (compared with free drugs, the inhibition activity of two drugs encapsulated in C + T/RBCm@Lip both decreased by a factor of 1.18). In addition to erythrocyte membranes, the coating of bovine serum albumin (BSA) also can endow ordinary liposomes with long circulation function. Wei et al. [48][69] prepared CUR liposomes with a BSA coating (BSA-CUR-Lips). It was found that the phagocytosis of BSA-CUR-Lips by the mouse macrophage Raw 264 was significantly reduced (p < 0.05), indicating that the liposomes could exert long-circulating effects. In addition, BSA-coated nano-carriers also have potential values for applications in bioimaging [49][70].
On the other hand, liposomes can be modified with structure-specific chemicals to obtain long-circulating effects. Utilizing 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy-PEG2000 (DSPE-PEG2000) to modify liposomes has become a research hotspot over the years. DSPE-PEG2000, causing powerful steric hindrance and hydrophilicity in liposome systems, can prevent liposomes from binding to plasma opsonin or being ingested by monocytes and macrophages [42][50][51][63,71,72]. Long-circulating liposomes are much more suitable for diseases that require long-term and frequent administration and therefore have promising applications in cancer chemotherapy.
Despite the long-circulating effect and better pharmacological efficacy of such liposome, high doses of cholesterol, which play the key role of membrane stabilization, have severely hindered the clinical application of liposome in cancer patients who also suffer from concomitant hyperlipidemia and cardia-cerebrovascular diseases [52][53][54][55][73,74,75,76]. Moreover, the use of cholesterol also refers to religion and vegetarianism [55][76]. Numerous methods have been explored to address these issues. β-sitosterol succinic anhydride ester, a potential alternative drug delivery carrier for cholesterol, was linked to PEG2000 and applied to the preparation of a gambogic acid liposome [42][63]. While possessing more outstanding long-term circulation effects than ordinary long-circulating liposomes (compared with the ordinary long-circulating liposomes, the t1/2 and AUC of the novel liposomes were increased by 12.5% and 47.1%, respectively), this novel long-circulating liposome could also remedy the deficiency of cholesterol.
Ginsenosides are a class of compounds with both hydrophilicity and hydrophobicity, in which the hydrophobic domain is equipped with the same steroid structure as cholesterol, and the hydrophilic domain is constituted by two glucose groups [56][57][77,78]. First of all, from the perspective of carrier structure, the ginsenosides Rg3 and Rb2 can not only substitute cholesterol for exerting a membrane stabilizing effect but also act as long-circulating stealther instead of DSPE-PEG2000 [55][58][76,79]. Additionally, in the sight of therapeutic effects, when combined with chemotherapy drugs, the ginsenosides Rg3 and Rb2 can exert synergistic anti-cancer effects [58][59][60][61][79,80,81,82].
It can be seen that the design of nanocarriers is constantly being updated, and numerous attempts have been made to design for safer and more effective ways to prepare nanocarriers with excellent characteristics such as high stability, long circulation, more powerful efficacy, and so on. Natural medicines have found their way here, which can be used not only as an alternative or synergist for traditional chemotherapy agent but also a pharmaceutical excipient in the production of anti-cancer preparation. The exploration of nano-preparation of natural medicines is meaningful and worthy of further study.
1.3. Nanoemulsion
Nanoemulsions are thermodynamically stable colloidal solutions formed by droplets of the internal phase, with a particle size of 50 to 100 nm dispersed in in the external phase [62][83]. There are two major administration routes in the use of nanoemulsion: transdermal administration and oral administration [63][64][84,85]. As an ideal drug delivery carrier, nanoemulsions can increase the solubility, bioavailability, and anti-tumor activities of drugs [65][86]. At present, there are three main ways for nanoemulsions to carry natural medicines. Firstly, monomeric compounds with anti-tumor activities isolated from natural medicines were directly encapsulated using nanoemulsion technology [66][67][87,88]. In one study, luteolin was encapsulated into a nanoemulsion for the treatment of breast cancer. Due to its ability to improve the permeability of the skin stratum corneum, such nanoemulsions could directly deliver drugs to the tumor site through transdermal administration [66][87]. Secondly, aqueous solutions of polysaccharides in natural medicines are usually used as aqueous phases to prepare nanoemulsions. Li et al. [64][85] prepared a water-in-oil (W/O) nanoemulsion of shiitake mushroom polysaccharide (SMP), which was able to maintain relatively stable droplet size for 3 months (storage conditions were 4 °C or 37 °C), and the intestinal absorption and anti-tumor activity of SMP were clearly improved (anti-tumor activity for 18-fold, compared to non-treated SMP). Thirdly, using essential oils of natural medicines as the oil phase to prepare a nanoemulsion can not only improve the solubility and bio-accessibility of an essential oil, fully exert its anti-tumor effects, and expand its application range, but also avoid the potential toxicity caused by conventional oil phases [68][69][70][89,90,91]. Alam et al. [70][91] prepared a nanoemulsion system with high stability using cinnamon essential oil both as antineoplastic agent and as oil phase. Compared with cinnamon essential oil, the cytotoxic effect of nanoemulsion against A549 cells was significantly improved, with the IC50 decreased by a factor of 2.77.
In addition, compared with other forms of nanoemulsions, there are few studies on the preparation of nanoemulsions from aqueous polysaccharide solutions. It has been reported that polysaccharides in other natural medicines also possessed anti-tumor effects, such as Angelica sinensis polysaccharides [71][92], Dendrobium wardianum polysaccharides [72][93], and Poria cocos polysaccharides [73][94]. However, few researchers have made them into nanoemulsions. Therefore, it may be a novel and promising research direction to prepare nanoemulsions using polysaccharides in natural medicines, which is expected to expand the application scope of polysaccharides and improve their anti-tumor activities.
2. Active Ingredients in Natural Product-Mediated Drug Targeting
In natural medicines, there is a subset of drugs that are similar to targeting formulations in modern medicine and normally used to achieve “site-directed” effects [74][100]. As shown in Figure 1, a drug transportation system modified with active ingredients of natural medicines could change the action site of other drugs and increase the distribution of drugs in targeted tissues, which have important application values in nano-formulations.
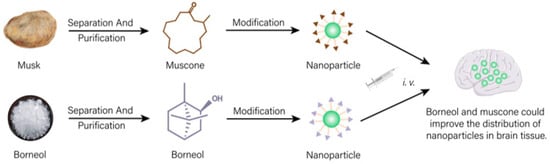
Figure 1.
Drug delivery system modified with effective ingredients of natural medicines could increase distribution of other drugs in targeted tissue.
Borneol and musk, belonging to aromatic resuscitation herbs in natural medicines, can be used to modify nano-preparations acting on the brain, which can increase drug distribution in the brain and improve the brain targeting of the formulation [75][76][77][101,102,103]. Borneol (Bor) and transmembrane peptide Pep-1 co-modified micelles loaded with carmostine exhibited good therapeutic effects on brain glioma. Bor could effectively improve the blood–brain barrier (BBB) permeability for drugs. Cell experiments showed that the modification of Bor could not only remarkably enhance the cytotoxicity of drugs on human glioma BT325 cells (p < 0.01) but also evidently increase the uptake of micelles by brain microvascular endothelial cell lines HBMEC (fluorescence intensity increased 1.67 times). In vivo experiments showed that the fluorescence of untargeted micelles labeled with fluorescent probe DiD disappeared rapidly at 6 h post-injection. A single dose of Bor-modified micelles significantly increased the signal distribution in brain tissue (p < 0.01), suggesting that Bor modification enhanced its ability to penetrate the BBB. Additionally, the signal was still observed 24 h after treatment, indicating prolonged retention times in in brain tissue [75][101]. Other studies showed that similar to Bor, due to the brain targeting property of muscone, doxorubicin-loaded liposomes modified with muscone could cross the BBB. Compared with unmodified liposomes, muscone-modified liposomes showed concentrated accumulation in the glioma region of the brain and less distribution outside the glioma region. Thus, muskone modification could increase the distribution of drugs in brain tissue, then improving the curative effect for brain glioma of antineoplastic drug [77][103].
3. Ligand-Mediated Drug Targeting
3.1. APRPG Peptide Modified Nanocarrier
Vascular endothelial growth factor (VEGF) is highly expressed on tumor vascular endothelial cells but rarely on normal endothelial cells [78][79][104,105], making it a desirable target point for anti-tumor drug delivery. Ala-Pro-Arg-Pro-Gly (APRPG), the small molecule peptide sequence, is able to specifically bind to VEGF receptors (VEGFR). The modification of APRPG on the nano-formulations can considerably increase the effect of the first-level drug targeting by means of active targeting. It can actively deliver the drug to the tumor tissue and improve the effectiveness of chemotherapy [80][81][82][106,107,108]. After intravenous administration, APRPG-modified nanoparticles loaded with PTX and norethindrone were concentrated at the cancer site of mice and effectively inhibited the growth of ectopic solid tumors in tumor-bearing mice (the inhibition rate of APRPPG-modified nanoparticles group and non-targeted nanoparticles group were 78.67% and 62.98%, respectively) (p < 0.01), suggesting that APRPG could deliver chemotherapeutic drugs more effectively to tumor tissues through active targeting [82][108].
It is important to note that VEGF is not only a target for drug delivery but also the site of action of natural anti-tumor monomer components. For example, triptolide restrained breast cancer cell angiogenesis through inhibiting the ERK1/2-HIF1-α-VEGFA axis [83][109]. Honokiol inhibited the NF-κB pathway, which, in turn, led to the down-regulation of VEGF expression and reduced the viability and angiogenesis of human lung cancer cell lines [84][110]. Cantharidin inhibited tumor angiogenesis by suppressing VEGF-induced signaling pathways [85][111]. The development of angiogenesis inhibitors targeting VEGF/VEGFR has become a vital field in anti-tumor research.
3.2. NGR Peptide-Modified Nanocarrier
NGR peptide (NGR), a peptide containing an asparagine-glycine-arginine (Asn-Gly-Arg) motif, is capable of specifically recognizing aminopeptidase N (APN/CD13), which is highly expressed on tumor vascular cells [86][87][88][112,113,114]. NGR is considered as a potential targeting ligand that can target tumor blood vessels [86][112]. Therefore, similar to APRPG, NGR ligands can be used for surface modifications of nano-formulations to enhance the targeting ability of drug delivery systems to tumor tissues [89][90][115,116].
This targeted ligand can not only increase the effect of first-level drug targeting depending on the means of active targeting, so that chemotherapeutic drugs or nano-agents with anti-tumor effects can accumulate more effectively and selectively in cancer tissues and thus be more fully taken up by tumor cells. It can also make drugs that have the ability to regulate the tumor environment accurately locate in tumor tissue and act as an anti-tumor agent indirectly by improving the tumor environment. A dual-targeted micelle-liposome bilayer delivery platform triggered by matrix metalloproteinases was designed by Duan et al. [89][115] for simultaneous loading of the anti-fibrotic drug quercetin (Que) and the herbal chemotherapeutic drug PTX. Owing to the first-level drug targeting function of NGR, the liposomes carrying Que on the outer layer of the formulation specifically accumulate at the tumor tissue to exert the anti-fibrotic effect of Que and ameliorate the tumor microenvironment. Modification of NGR evidently facilitated intracellular accumulation of liposomes in human umbilical vein cell lines (HUVEC), with an uptake 1.3 times higher than that of unmodified liposomes. This suggested that the NGR-modified formulation could function to target tumor tissue through CD13 receptor-mediated endocytosis. The results of in vivo experiments exhibited that non-targeted agents were rapidly eliminated after injection, whereas NGR modification not only significantly increased the accumulation of the agents at the cancer site (p < 0.05) but also distinctly prolonged the residence time of nanoparticles at the tumor site (fluorescent signals were still detectable after intravenous injection for 24 h), suggesting that NGR played a crucial role in mediating the accumulation of nano-agents in tumor tissue.
It is worth noting that the above two targets and pathways initially are not used for the first-level drug targeting but for specifically delivering anti-angiogenic drugs to tumor blood vessels for the purpose of tumor therapy by inhibiting the generation of new blood vessels [86][91][112,117]. But now, NGR and APRPG have found their new way to achieve a tumor tissue-specific targeting function as an active targeting ligand. This also provides inspiration and ideas for future research: due to the difficulty in discovering new targets and pathways, when designing new targeting carriers, the flexibility of a nanocarrier can be fully utilized. Starting from known targets and pathways, new carrier forms can be explored to obtain more novel and powerful targeting strategies and expand the application range of natural medicines’ nano-formulations.
