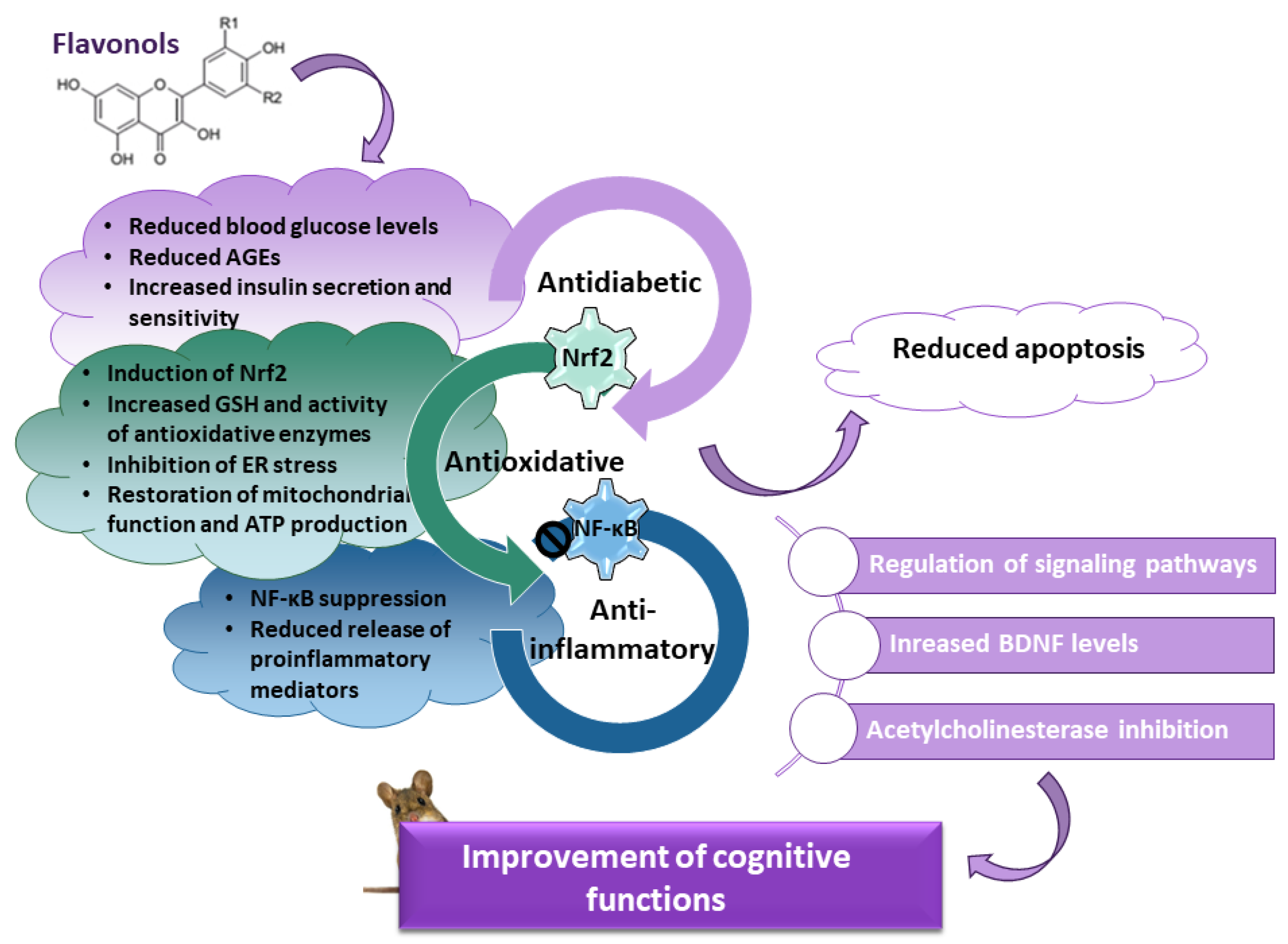
Diabetes mellitus is a complex metabolic disease associated with reduced synaptic plasticity, atrophy of the hippocampus, and cognitive decline. Cognitive impairment results from several pathological mechanisms, including increased levels of advanced glycation end products (AGEs) and their receptors, prolonged oxidative stress and impaired activity of endogenous mechanisms of antioxidant defense, neuroinflammation driven by the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB), decreased expression of brain-derived neurotrophic factor (BDNF), and disturbance of signaling pathways involved in neuronal survival and cognitive functioning. Flavonols, a highly abundant class of flavonoids in the human diet, are appreciated as a potential pharmacological intervention against cognitive decline in diabetes.
- diabetes
- cognitive functions
- flavonols
- oxidative stress
1. Introduction
2. Mechanisms Underlying the Beneficial Effects of Quercetin in Diabetic Animals
3. Effects of Other Flavonols on Diabetes-Induced Cognitive Decline in Animal Models
| Flavonol | Experimental Model | Route of Administration, Dose, and Treatment Duration | The Mechanisms Contributing to the Improvement of Cognitive Functions in the Neuronal Tissue | Reference(s) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Quercetin | STZ-induced diabetic rats | p.o., 5, 25 and 50 mg/kg/day, for 40 days |
↓ MDA levels ↓ ADA activity ↓ AChE activity ↑ NTPDase activity |
[239] | [37] | ||||
| female diabetic ( | db | / | db | ) mice | p.o., 70 mg/kg/day, for 12 weeks |
↑ expression of synapse-related proteins (PSD93, PSD95) ↑ neurotrophic factors (BDNF, NGF) ↑ SIRT1 protein expression ↓ expression of ER stress markers (PERK, IRE-1α, ATF6, eIF2α, BIP, and PDI) ↓ oxidative stress levels |
[240] | [38] | |
| STZ-induced diabetic rats | p.o., 30 and 60 mg/kg/day, for 6 weeks |
restoration of the mitochondrial energy metabolism ↑ ATP production ↑ pAMPK, PGC-1α, SIRT1, NRF1, and TFAM expression ↑ AMPK/PGC-1α pathway |
[236] | [34] | |||||
| High-fat diet- and STZ-induced diabetic rats | i.p., 50 mg/kg/day, for 14 days |
↓ P2X | 4 | receptor expression ↓ P2X | 4 | and GFAP coexpression ↓ p38MAPK pathway ↓ p-p38MAPK |
[243] | [41] | |
| STZ-induced diabetic rats | p.o., 25 mg/kg/day quercetin or QCSPIONs, for 35 days |
normalized total antioxidant capacity ↓ miR-27a expression ↑ Nrf2 and expression of responsive antioxidant genes |
[229,245] | [25][43] | |||||
| Diabetic ( | db | / | db | ) mice |
p.o., 70 mg/kg/day, for 12 weeks |
↑ expression of synapse-related proteins (PSD93, PSD95) ↑ neurotrophic factors (BDNF, NGF) ↑ SIRT1 expression ↓ expression of NLRP3 inflammation- related proteins ↓ NLRP3, adaptor protein ASC ↓ cleaved caspase-1, ↓ expression of pro-inflammatory cytokines IL-1β and IL-18 ↓ NLRP3 inflammasome activation ↓ expression of proapoptotic proteins |
[246] | [44] | |
| Rutin | STZ-induced diabetic rats | p.o., 100 mg/kg/day, for 5 weeks |
↑ BDNF and NGF levels ↑ GSH levels ↓ lipid peroxidation antiapoptotic effect ↓ caspase-3 levels ↑ Bcl-2 |
[262] | [62] | ||||
| Troxerutin | STZ-induced diabetic rats | p.o., 150 mg/kg/day, for 6 weeks |
↓ lipid peroxidation ↓ oxidative stress ↑ SOD activity ↓ expression of NADPH oxidase subunits ↑ nuclear translocation of Nrf2 ↑ cytosolic fraction of HO-1 and NQO1 |
[263] | [63] | ||||
| STZ-induced diabetic rats | i.p., 60 mg/kg/day, for 6 weeks |
↑ SOD activity ↑ GSH level ↑ GCLM and GCLC subunits expression ↓ MDA level |
[264] | [64] | |||||
| STZ-induced diabetic rats | i.p., 60 mg/kg/day, for 12 weeks |
↑ Nrf2 expression ↑ SOD activity ↓ lipid peroxidation |
[265] | [65] | |||||
| Myricetin | STZ-induced diabetic rats | i.p., 0.5, 1 or 2 mg/kg/day, for 2 weeks |
↓ generation of AGEs and ROS ↑ Na+, K+-ATPase activity ↑ activity of antioxidative enzymes ↑ H | 2 | S, HO-1 and Nrf2 levels ↑ Nrf2 pathway |
[278] | [78] | ||
| STZ-induced diabetic rats | i.p., 5 or 10 mg/kg/day, for 21 day |
↑ number of hippocampal CA3 pyramidal neurons | [279] | [79] | |||||
| Dihydromyricetin | High-sugar, high-fat, and STZ-induced diabetic mice | p.o., 125 or 250 mg/kg/day, for 16 weeks |
↓ oxidative stress ↓ MDA accumulation ↑ SOD, catalase and GPx ↑ BDNF |
[284] | [84] | ||||
| Morin | STZ-induced diabetic rats | 50 mg/kg/day, for 60 days |
↓ oxidative damage of proteins and membrane lipids ↓ apoptosis ↑ BDNF levels ↑ TrkB/Akt pathway |
[306] | [106] |
References
- Sharma, G.; Parihar, A.; Talaiya, T.; Dubey, K.; Porwal, B.; Parihar, M.S. Cognitive impairments in type 2 diabetes, risk factors and preventive strategies. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190105.
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300.
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497.
- Szwajgier, D. Anticholinesterase activities of selected polyphenols—A short report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64.
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jazvinšćak Jembrek, M. PI3K/Akt and ERK1/2 Signalling Are Involved in Quercetin-Mediated Neuroprotection against Copper-Induced Injury. Oxidative Med. Cell. Longev. 2020, 2020, 9834742.
- Jazvinšćak Jembrek, M.; Oršolić, N.; Mandić, L.; Sadžak, A.; Šegota, S. Anti-Oxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Flavonols: Targeting Nrf2, NF-κB and p53 Pathways in Neurodegeneration. Antioxidants 2021, 10, 1628.
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717.
- Wang, L.; Lee, I.M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009, 89, 905–912.
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252.
- Sebastian, R.S.; Wilkinson Enns, C.; Goldman, J.D.; Martin, C.L.; Steinfeldt, L.C.; Murayi, T.; Moshfegh, A.J.A. New Database Facilitates Characterization of Flavonoid Intake, Sources, and Positive Associations with Diet Quality among US Adults. J. Nutr. 2015, 145, 1239–1248.
- Yammine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.J.; Lizard, G.; Latruffe, N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules 2021, 26, 3483.
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951.
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133.
- Hollman, P.C.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food. Chem. Toxicol. 1999, 37, 937–942.
- Vasilopoulou, E.; Georga, K.; Joergensen, M.; Naska, A.; Trichopoulou, A. The antioxidant properties of Greek foods and the flavonoid content of the Mediterranean menu. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 2005, 5, 33–45.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S. Food Flavonols: Nutraceuticals with Complex Health Benefits and Functionalities. Trends Food Sci. Technol. 2021, 117, 194–204.
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060.
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814.
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750.
- Svilaas, A.; Sakhi, A.K.; Andersen, L.F.; Svilaas, T.; Ström, E.C.; Jacobs, D.R., Jr.; Ose, L.; Blomhoff, R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J. Nutr. 2004, 134, 562–567.
- Hamer, M.; Chida, Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and meta-analysis. J. Hypertens. 2007, 25, 2361–2369.
- Dauchet, L.; Péneau, S.; Bertrais, S.; Vergnaud, A.C.; Estaquio, C.; Kesse-Guyot, E.; Czernichow, S.; Favier, A.; Faure, H.; Galan, P.; et al. Relationships between different types of fruit and vegetable consumption and serum concentrations of antioxidant vitamins. Br. J. Nutr. 2008, 100, 633–641.
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091.
- Bacchetti, T.; Turco, I.; Urbano, A.; Morresi, C.; Ferretti, G. Relationship of fruit and vegetable intake to dietary antioxidant capacity and markers of oxidative stress: A sex-related study. Nutrition 2019, 61, 164–172.
- Perrig, W.J.; Perrig, P.; Stähelin, H.B. The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 1997, 45, 718–724.
- Tuzcu, M.; Baydas, G. Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur. J. Pharmacol. 2006, 537, 106–110.
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Bhutada, C.; Tawari, S.; Dixit, P.; Mundhada, D. Ameliorative effect of quercetin on memory dysfunction in streptozotocin-induced diabetic rats. Neurobiol. Learn. Mem. 2010, 94, 293–302.
- Root, M.; Ravine, E.; Harper, A. Flavonol Intake and Cognitive Decline in Middle-Aged Adults. J. Med. Food 2015, 18, 1327–1332.
- Holland, T.M.; Agarwal, P.; Wang, Y.; Dhana, K.; Leurgans, S.E.; Shea, K.; Booth, S.L.; Rajan, K.B.; Schneider, J.A.; Barnes, L.L. Association of Dietary Intake of Flavonols with Changes in Global Cognition and Several Cognitive Abilities. Neurology 2023, 100, e694–e702.
- Jacques, P.F.; Cassidy, A.; Rogers, G.; Peterson, J.J.; Meigs, J.B.; Dwyer, J.T. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J. Nutr. 2013, 143, 1474–1480.
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417.
- Jazvinšćak Jembrek, M.; Oršolić, N.; Karlović, D.; Peitl, V. Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 6888.
- Yao, Z.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; Sun, S.; et al. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur. J. Nutr. 2019, 58, 819–830.
- Mahesh, T.; Menon, V.P. Quercetin alleviates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004, 18, 123–127.
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The effect of hesperidin and quercetin on oxidative stress, NF-κB and SIRT1 levels in a STZ-induced experimental diabetes model. Biomed. Pharmacother. 2017, 90, 500–508.
- Ebrahimpour, S.; Esmaeili, A.; Beheshti, S. Effect of quercetin-conjugated superparamagnetic iron oxide nanoparticles on diabetes-induced learning and memory impairment in rats. Int. J. Nanomed. 2018, 13, 6311–6324.
- Dini, S.; Zakeri, M.; Ebrahimpour, S.; Dehghanian, F.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles modulate glucose metabolism-related genes and miR-29 family in the hippocampus of diabetic rats. Sci. Rep. 2021, 11, 8618.
- Kannappan, S.; Anuradha, C.V. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J. Med. Res. 2009, 129, 401–408.
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146.
- Tota, S.; Awasthi, H.; Kamat, P.K.; Nath, C.; Hanif, K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav. Brain Res. 2010, 209, 73–79.
- Chiş, I.C.; Mureşan, A.; Oros, A.; Nagy, A.L.; Clichici, S. Protective effects of Quercetin and chronic moderate exercise (training) against oxidative stress in the liver tissue of streptozotocin-induced diabetic rats. Physiol. Int. 2016, 103, 49–64.
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582.
- Zhang, Q.; Song, W.; Zhao, B.; Xie, J.; Sun, Q.; Shi, X.; Yan, B.; Tian, G.; Liang, X. Quercetin Attenuates Diabetic Peripheral Neuropathy by Correcting Mitochondrial Abnormality via Activation of AMPK/PGC-1α Pathway in vivo and in vitro. Front. Neurosci. 2021, 15, 636172.
- El-Shaer, N.O.; Hegazy, A.M.; Muhammad, M.H. Protective effect of quercetin on pulmonary dysfunction in streptozotocin-induced diabetic rats via inhibition of NLRP3 signaling pathway. Environ. Sci. Pollut. Res. Int. 2023, 30, 42390–42398.
- Rahmani, A.H.; Alsahli, M.A.; Khan, A.A.; Almatroodi, S.A. Quercetin, a Plant Flavonol Attenuates Diabetic Complications, Renal Tissue Damage, Renal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Metabolites 2023, 13, 130.
- Maciel, R.M.; Carvalho, F.B.; Olabiyi, A.A.; Schmatz, R.; Gutierres, J.M.; Stefanello, N.; Zanini, D.; Rosa, M.M.; Andrade, C.M.; Rubin, M.A.; et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: Role of ectonucleotidases and acetylcholinesterase activities. Biomed. Pharmacother. 2016, 84, 559–568.
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.B.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015–7029.
- Teixeira, J.M.; Dos Santos, G.G.; Neves, A.F.; Athie, M.C.P.; Bonet, I.J.M.; Nishijima, C.M.; Farias, F.H.; Figueiredo, J.G.; Hernandez-Olmos, V.; Alshaibani, S.; et al. Diabetes-induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia. Neuroscience 2019, 398, 158–170.
- Shi, Y.; Liang, X.C.; Zhang, H.; Wu, Q.L.; Qu, L.; Sun, Q. Quercetin protects rat dorsal root ganglion neurons against high glucose-induced injury in vitro through Nrf-2/HO-1 activation and NF-κB inhibition. Acta Pharmacol. Sin. 2013, 34, 1140–1148.
- Yang, R.; Li, L.; Yuan, H.; Liu, H.; Gong, Y.; Zou, L.; Li, S.; Wang, Z.; Shi, L.; Jia, T.; et al. Quercetin relieved diabetic neuropathic pain by inhibiting upregulated P2X4 receptor in dorsal root ganglia. J. Cell. Physiol. 2019, 234, 2756–2764.
- Zhou, X.Y.; Ying, C.J.; Hu, B.; Zhang, Y.S.; Gan, T.; Zhu, Y.D.; Wang, N.; Li, A.A.; Song, Y.J. Receptor for advanced glycation end products aggravates cognitive deficits in type 2 diabetes through binding of C-terminal AAs 2-5 to mitogen-activated protein kinase kinase 3 (MKK3) and facilitation of MEKK3-MKK3-p38 module assembly. Aging Cell 2022, 21, e13543.
- Farhadi, A.; Totonchi, M.; Nabavi, S.M.; Baharvand, H.; Pakdaman, H.; Hajizadeh-Saffar, E.; Mousavi, S.A.; Hadi, F.; Al-Sinawi, H.; Li, Q.; et al. P38 initiates degeneration of midbrain GABAergic and glutamatergic neurons in diabetes models. Eur. J. Neurosci. 2022, 56, 3755–3778.
- Ebrahimpour, S.; Esmaeili, A.; Dehghanian, F.; Beheshti, S. Effects of quercetin-conjugated with superparamagnetic iron oxide nanoparticles on learning and memory improvement through targeting microRNAs/NF-κB pathway. Sci. Rep. 2020, 10, 15070.
- Ebrahimpour, S.; Shahidi, S.B.; Abbasi, M.; Tavakoli, Z.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci. Rep. 2020, 10, 15957.
- Hu, T.; Lu, X.-Y.; Shi, J.-J.; Liu, X.-Q.; Chen, Q.-B.; Wang, Q.; Chen, Y.-B.; Zhang, S.-J. Quercetin protects against diabetic encephalopathy via SIRT1/NLRP3 pathway in db/db mice. J. Cell. Mol. Med. 2020, 24, 3449–3459.
- Ying, L.; Chaudhry, M.T.; Xiao, F.; Mao, Y.; Wang, M.; Wang, B.; Wang, S.; Li, Y. The Effects and Mechanism of Quercetin Dietary Supplementation in Streptozotocin-Induced Hyperglycemic Arbor Acre Broilers. Oxidative Med. Cell. Longev. 2020, 2020, 9585047.
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340.
- Mazloom, Z.; Abdollahzadeh, S.M.; Dabbaghmanesh, M.-H.; Rezaianzadeh, A. The effect of quercetin supplementation on oxidative stress, glycemic control, lipid profile and insulin resistance in type 2 diabetes: A randomized clinical trial. J. Health Sci. Surveill. Syst. 2014, 2, 8–14.
- Nishihira, J.; Nishimura, M.; Kurimoto, M.; Kagami-Katsuyama, H.; Hattori, H.; Nakagawa, T.; Muro, T.; Kobori, M. The effect of 24-week continuous intake of quercetin-rich onion on age-related cognitive decline in healthy elderly people: A randomized, double-blind, placebo-controlled, parallel-group comparative clinical trial. J. Clin. Biochem. Nutr. 2021, 69, 203–215.
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khuwaja, G.; Srivastawa, P.; Khan, M.B.; Raza, S.S.; Javed, H.; Vaibhav, K.; Khan, A.; et al. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009, 1292, 123–135.
- Koda, T.; Kuroda, Y.; Imai, H. Rutin supplementation in the diet has protective effects against toxicant-induced hippocampal injury by suppression of microglial activation and pro-inflammatory cytokines: Protective effect of rutin against toxicant-induced hippocampal injury. Cell. Mol. Neurobiol. 2009, 29, 523–531.
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034.
- Stanley Mainzen Prince, P.; Kamalakkannan, N. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Mol. Toxicol. 2006, 20, 96–102.
- Kamalakkannan, N.; Prince, P.S. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103.
- Kamalakkannan, N.; Stanely Mainzen Prince, P. Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol. Cell. Biochem. 2006, 293, 211–219.
- Fernandes, A.A.; Novelli, E.L.; Okoshi, K.; Okoshi, M.P.; Di Muzio, B.P.; Guimarães, J.F.; Fernandes Junior, A. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219.
- Butchi Akondi, R.; Kumar, P.; Annapurna, A.; Pujari, M. Protective Effect of Rutin and Naringin on Sperm Quality in Streptozotocin (STZ) Induced Type 1 Diabetic Rats. Iran. J. Pharm. Res. 2011, 10, 585–596.
- Niture, N.T.; Ansari, A.A.; Naik, S.R. Anti-hyperglycemic activity of rutin in streptozotocin-induced diabetic rats: An effect mediated through cytokines, antioxidants and lipid biomarkers. Indian J. Exp. Biol. 2014, 52, 720–727.
- Liang, W.; Zhang, D.; Kang, J.; Meng, X.; Yang, J.; Yang, L.; Xue, N.; Gao, Q.; Han, S.; Gou, X. Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomed. Pharmacother. 2018, 107, 721–728.
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 210, 340–352.
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective Effects of Rutin in Streptozotocin-Induced Diabetic Rat Retina. J. Mol. Neurosci. 2015, 56, 440–448.
- Gao, M.; Kang, Y.; Zhang, L.; Li, H.; Qu, C.; Luan, X.; Liu, L.; Zhang, S. Troxerutin attenuates cognitive decline in the hippocampus of male diabetic rats by inhibiting NADPH oxidase and activating the Nrf2/ARE signaling pathway. Int. J. Mol. Med. 2020, 46, 1239–1248.
- Zhang, S.; Li, H.; Zhang, L.; Li, J.; Wang, R.; Wang, M. Effects of troxerutin on cognitive deficits and glutamate cysteine ligase subunits in the hippocampus of streptozotocin-induced type 1 diabetes mellitus rats. Brain Res. 2017, 1657, 355–360.
- Zhang, S.; Yuan, L.; Zhang, L.; Li, C.; Li, J. Prophylactic Use of Troxerutin Can Delay the Development of Diabetic Cognitive Dysfunction and Improve the Expression of Nrf2 in the Hippocampus on STZ Diabetic Rats. Behav. Neurol. 2018, 2018, 8678539.
- Yavari, R.; Badalzadeh, R.; Alipour, M.R.; Tabatabaei, S.M. Modulation of hippocampal gene expression of microRNA-146a/microRNA-155-nuclear factor-kappa B inflammatory signaling by troxerutin in healthy and diabetic rats. Indian J. Pharmacol. 2016, 48, 675–680.
- Hoseindoost, M.; Alipour, M.R.; Farajdokht, F.; Diba, R.; Bayandor, P.; Mehri, K.; Nayebi Rad, S.; Babri, S. Effects of troxerutin on inflammatory cytokines and BDNF levels in male offspring of high-fat diet fed rats. Avicenna J. Phytomed. 2019, 9, 597–605.
- Zamanian, M.; Bazmandegan, G.; Sureda, A.; Sobarzo-Sanchez, E.; Yousefi-Manesh, H.; Shirooie, S. The Protective Roles and Molecular Mechanisms of Troxerutin (Vitamin P4) for the Treatment of Chronic Diseases: A Mechanistic Review. Curr. Neuropharmacol. 2021, 19, 97–110.
- Taheri, Y.; Suleria, H.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241.
- Liu, I.M.; Liou, S.S.; Lan, T.W.; Hsu, F.L.; Cheng, J.T. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2005, 71, 617–621.
- Ozcan, F.; Ozmen, A.; Akkaya, B.; Aliciguzel, Y.; Aslan, M. Beneficial effect of myricetin on renal functions in streptozotocin-induced diabetes. Clin. Exp. Med. 2012, 12, 265–272.
- Li, Y.; Ding, Y. Minireview: Therapeutic potential of myricetin in diabetes mellitus. Food Sci. Hum. Wellness 2012, 1, 19–25.
- Kandasamy, N.; Ashokkumar, N. Protective effect of bioflavonoid myricetin enhances carbohydrate metabolic enzymes and insulin signaling molecules in streptozotocin–cadmium induced diabetic nephrotoxic rats. Toxicol. Appl. Pharmacol. 2014, 279, 173–185.
- Niisato, N.; Marunaka, Y. Therapeutic potential of multifunctional myricetin for treatment of type 2 diabetes mellitus. Front. Nutr. 2023, 10, 1175660.
- Al-Abbasi, F.A.; Kazmi, I. Therapeutic role of kaempferol and myricetin in streptozotocin-induced diabetes synergistically via modulation in pancreatic amylase, glycogen storage and insulin secretion. Mol. Cell. Biochem. 2023, 478, 1927–1937.
- Li, Y.; Zheng, X.; Yi, X.; Liu, C.; Kong, D.; Zhang, J.; Gong, M. Myricetin: A potent approach for the treatment of type 2 diabetes as a natural class B GPCR agonist. FASEB J. 2017, 31, 2603–2611.
- Liu, I.M.; Liou, S.S.; Cheng, J.T. Mediation of beta-endorphin by myricetin to lower plasma glucose in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2006, 104, 199–206.
- Ma, J.; Liu, J.; Chen, Y.; Yu, H.; Xiang, L. Myricetin Improves Impaired Nerve Functions in Experimental Diabetic Rats. Front. Endocrinol. 2022, 13, 915603.
- Ramezani, M.; Darbandi, N.; Khodagholi, F.; Hashemi, A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer’s disease. Neural Regen. Res. 2016, 11, 1976–1980.
- Shimada, Y.; Sato, Y.; Kumazoe, M.; Kitamura, R.; Fujimura, Y.; Tachibana, H. Myricetin improves cognitive function in SAMP8 mice and upregulates brain-derived neurotrophic factor and nerve growth factor. Biochem. Biophys. Res. Commun. 2022, 616, 33–40.
- Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites 2022, 13, 17.
- Philbert, S.A.; Schönberger, S.J.; Xu, J.; Church, S.J.; Unwin, R.D.; Cooper, G.J.S. Elevated hippocampal copper in cases of type 2 diabetes. EBioMedicine 2022, 86, 104317.
- Sadžak, A.; Vlašić, I.; Kiralj, Z.; Batarelo, M.; Oršolić, N.; Jazvinšćak Jembrek, M.; Kušen, I.; Šegota, S. Neurotoxic effect of flavonol myricetin in the presence of excess copper. Molecules 2021, 26, 845.
- Ling, H.; Zhu, Z.; Yang, J.; He, J.; Yang, S.; Wu, D.; Feng, S.; Liao, D. Dihydromyricetin improves type 2 diabetes-induced cognitive impairment via suppressing oxidative stress and enhancing brain-derived neurotrophic factor-mediated neuroprotection in mice. Acta Biochim. Biophys. Sin. 2018, 50, 298–306.
- Zhang, X.; Zhang, K.; Wang, Y.; Ma, R. Effects of Myricitrin and Relevant Molecular Mechanisms. Curr. Stem Cell Res. Ther. 2020, 15, 11–17.
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Solid Lipid Nanoparticles of Myricitrin Have Antioxidant and Antidiabetic Effects on Streptozotocin-Nicotinamide-Induced Diabetic Model and Myotube Cell of Male Mouse. Oxidative Med. Cell. Longev. 2018, 2018, 7496936.
- Kim, D.Y.; Kim, S.R.; Jung, U.J. Myricitrin Ameliorates Hyperglycemia, Glucose Intolerance, Hepatic Steatosis, and Inflammation in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Int. J. Mol. Sci. 2020, 21, 1870.
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288.
- Babaei, P.; Eyvani, K.; Kouhestani, S. Sex-Independent Cognition Improvement in Response to Kaempferol in the Model of Sporadic Alzheimer’s Disease. Neurochem. Res. 2021, 46, 1480–1486.
- Silva Dos Santos, J.; Gonçalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700.
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338.
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209.
- Suchal, K.; Malik, S.; Khan, S.I.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Ojha, S.; Arya, D.S. Molecular Pathways Involved in the Amelioration of Myocardial Injury in Diabetic Rats by Kaempferol. Int. J. Mol. Sci. 2017, 18, 1001.
- Alshehri, A.S.; El-Kott, A.F.; Eleawa, S.M.; El-Gerbed, M.S.A.; Khalifa, H.S.; El-Kenawy, A.E.; Albadrani, G.M.; Abdel-Daim, M.M. Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J. Physiol. Pharmacol. 2021, 72, 339–355.
- Sheng, H.; Zhang, D.; Zhang, J.; Zhang, Y.; Lu, Z.; Mao, W.; Liu, X.; Zhang, L. Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front. Med. 2022, 9, 986825.
- Alshehri, A.S. Kaempferol attenuates diabetic nephropathy in streptozotocin-induced diabetic rats by a hypoglycaemic effect and concomitant activation of the Nrf-2/Ho-1/antioxidants axis. Arch. Physiol. Biochem. 2023, 129, 984–997.
- Abo-Salem, O.M. Kaempferol Attenuates the Development of Diabetic Neuropathic Pain in Mice: Possible Anti-Inflammatory and Anti-Oxidant Mechanisms. Open Access Maced. J. Med. Sci. 2014, 2, 424–430.
- Kishore, L.; Kaur, N.; Singh, R. Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology 2018, 26, 993–1003.
- Zhao, X.; Li, X.L.; Liu, X.; Wang, C.; Zhou, D.S.; Ma, Q.; Zhou, W.H.; Hu, Z.Y. Antinociceptive effects of fisetin against diabetic neuropathic pain in mice: Engagement of antioxidant mechanisms and spinal GABAA receptors. Pharmacol. Res. 2015, 102, 286–297.
- Sandireddy, R.; Yerra, V.G.; Komirishetti, P.; Areti, A.; Kumar, A. Fisetin Imparts Neuroprotection in Experimental Diabetic Neuropathy by Modulating Nrf2 and NF-κB Pathways. Cell. Mol. Neurobiol. 2016, 36, 883–892.
- Prasath, G.S.; Sundaram, C.S.; Subramanian, S.P. Fisetin averts oxidative stress in pancreatic tissues of streptozotocin-induced diabetic rats. Endocrine 2013, 44, 359–368.
- Currais, A.; Farrokhi, C.; Dargusch, R.; Armando, A.; Quehenberger, O.; Schubert, D.; Maher, P. Fisetin Reduces the Impact of Aging on Behavior and Physiology in the Rapidly Aging SAMP8 Mouse. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 299–307.
- Bachewal, P.; Gundu, C.; Yerra, V.G.; Kalvala, A.K.; Areti, A.; Kumar, A. Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. BioFactors 2018, 44, 109–122.
- AlSharari, S.D.; Al-Rejaie, S.S.; Abuohashish, H.M.; Aleisa, A.M.; Parmar, M.Y.; Mohammed, M. A Ameliorative potential of morin in streptozotocin-induced neuropathic pain in rats. Trop. J. Pharm. Res. 2014, 13, 1429–1436.
- Shyma, R.L.; Mini, S. Neuroprotective effect of Morin via TrkB/Akt pathway against diabetes mediated oxidative stress and apoptosis in neuronal cells. Toxicol. Mech. Methods 2022, 32, 695–704.
