Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Theodoros Varzakas.
Probiotic encapsulation techniques can be categorized into two types: chemical (coacervation, ionic gelation, and molecular inclusion), and physical (spray drying, freeze drying, spray chilling, spray cooling, extrusion, fluidized bed drying, electrospraying, and electrospinning). Probiotic cells are between 1 and 5 μm in size and their viability must be maintained during the encapsulation process. The main encapsulation processes for probiotics are freeze drying, spray drying, ionic gelation, complex coacervation, electrospraying, and electrospinning.
- encapsulation
- bioactive ingredients
- probiotics
- nanoemulsions
1. Spray Drying
Spray drying is mainly used to protect the heat-sensitive prebiotics and comprises a cheap and simple dehydration process being used on an industrial scale [1]. It is also used as an encapsulation technique. Bioactives are encapsulated following dissolution, emulsification, or dispersion with an aqueous or organic solution containing the encapsulating agent, homogenization with or without the use of an emulsifier, and then suspension or spraying the mixture in a drying chamber. Dry powder spirals to the bottom of the container where it is collected following evaporation of the water in the chamber [2][3][4]. The process is described as follows: I first phase includes injection via a pump of the liquid formulation (i.e., the mixture containing the solution with the wall material and bioactives) along with spraying at the entrance of the drying chamber. There, a nozzle or vaporizer that emits a stream of hot air helps to convert it into tiny droplets [2][3].
Commonly used nozzles are divided into three types: centrifugal disc sprayer, pneumatic nozzles, and pressure nozzles. The first is easy to use, whereas pneumatic nozzles are less efficient and increase costs, and pressure nozzles are suitable for drying high-viscosity solutions [5]. This is why homogenization is carried out at the initial stage, since it creates a stable solution with a low viscosity, producing smaller droplets without air being included in the particle. A high-viscosity solution may cause the formation of elongated and large droplets. This negatively affects the drying rate and therefore the spraying process [2].
Evaporation of the liquid occurs instantaneously since the size of the droplets that have been created is extremely small, resulting in an increase in their contact surface with the hot air stream. Hence, the drying process is completed at a rapid rate [2][3]. It should be emphasized that the gas with which the droplets will come into contact takes the form of either heated atmospheric air or inert gas in the case of flammable or oxygen-sensitive drying products. Finally, the particles are collected at the bottom of the conical drying chamber where they are separated from the gas stream via filtration, while the gas is expelled outside the chamber [3].
Spray drying has proven to be an effective process for the relatively large particle size of probiotics [3][6]. Proteins, starches, polysaccharides, and sugars are usually used as wall materials. The membrane is protected by trehalose, maltodextrin, gum arabic, and whey protein, hence maintaining its integrity due to the formation of bonds with membrane proteins. The result of these properties is the control of moisture content and particle size [7]. During spray drying and according to Buchi apparatus, seven principles are adopted.
-
Heating of the inlet air to the desired temperature (<220 °C).
-
Two fluid nozzles operate the droplet formation, whereas an ultrasonic spray head operates the nano spray dryer.
-
Drying chamber (heat exchange between drying gas and droplets).
-
Particle collection using cyclones. Electrostatic particle collector for the nano spray dryer.
-
Outlet filter for the collection of the finest particles.
-
Drying gas is delivered by an aspirator. An aspirator or compressed air could be used by the nano spray dryer.
-
Filtering of the drying gas.
During spray drying, a thermally induced phase separation occurs at the surface of the droplet. This crust formation significantly decreases the diffusion coefficients of volatile organic compounds relative to water. The newly developed skin allows water to diffuse (selective diffusion), but most importantly, it aids the retention of flavor chemicals.
The species and strain of the probiotic, inlet temperature, outlet temperature, speed of atomization, osmotic stress, dehydration stress, and wall materials affect the probiotic survival rate [6][8][9]. Different studies include the addition of wall materials for probiotic viability preservation. Hydrolyzed WPI+FOS (1.5:1) maintained the viability of L. plantarum at >108 CFU/mL during 60 days of storage at 4 °C [10]. Similar behavior was described for skim milk, non-fat milk with xanthan gum, and WPI with dextran [11][12][13]. A viability of <109 CFU/g after 24 weeks of storage at 25 °C and 11% RH for microcapsules of L. rhamnosus with WPI (20%), inulin (4%), and Persian gum (1%) was reported by Moayyedi et al [14].
The buffering capacity of wall materials provides a good shield for probiotics, hence protecting them against GI tract conditions [9][11]. Occasionally, the acid and bile tolerance of some bacteria enhances their survival.
2. Spray Cooling
Spray cooling is similar to spray drying. In this method, particles are formed by cooling and solidification of the droplets and not by evaporation of the solvent. In spray cooling, energy is removed from the droplets, causing the core material to solidify, whereas in spray drying, energy is applied to the droplets, causing the solvent to evaporate [15][16]. The bioactives are then dispersed in a liquid matrix material. Lipids or gel-like hydrocolloids can be encapsulating materials with this method. Solidification of the matrix is induced around the dispersed bioactives following cold air or liquid nitrogen spraying in a chamber. This leads to the formation of microcapsules. The feed, air cooler, cooling chamber, product collector, and fan form the main parts of the spray cooling process [15][17].
The low cost, continuous process, suitability for industrial scale make spray cooling an advantageous technique for food ingredient encapsulation. In addition, the non-use of organic solvents makes it environmentally friendly. However, its operation at relatively high temperatures may not enable it to be a suitable option for heat-sensitive ingredients such as omega-3 fatty acids, certain enzymes, and probiotics. Moreover, the relatively low encapsulation efficiency and the losses of bioactives during storage might cause limitations of this method [15][17].
S. boulardii, L. acidophilus, and B. bifidum have been encapsulated using the spray chilling or cooling technique [18]. The survivability of spray-chilled (S. boulardii, 97.89%; L. acidophilus, 83.57%; B. bifidum, 88.50%) and spray-dried (S. boulardii, 97.51%; L. acidophilus, 84.05%; B. bifidum, 90.10%) probiotics under simulated gastric conditions showed a great similarity. Probiotics have been encapsulated through the spray chilling technique in probiotic-enriched cream-filled cakes [19] and savory cereal bars [20].
3. Freeze Drying or Lyophilization
Lyophilization is a dehydration method suitable for the encapsulation of thermally sensitive substances. This technique consists of three stages [21]. In the first stage, ingredients are frozen quickly and thus ice crystals are created in order to limit the damage to the cellular structure. The freezing must be complete because any water that is not frozen cannot be removed by sublimation. The quality of the freeze-dried product is affected by the speed of freezing. In liquid foods, for example, slow freezing is recommended, where large ice crystals are formed which are joined together in various places. In solid foods, rapid freezing is recommended, where numerous and small-sized crystals are formed which do not join together.
In a secondary phase, the removal of water and therefore the dehydration takes place in two stages: (a) through sublimation (moisture reduction to ~15%) and (b) via evaporation (desorption) of the non-frozen water (moisture reduction to ~2%). For sublimation dehydration, the frozen ingredient is introduced into the lyophilization dryer. A vacuum chamber, a product heating system, and a water vapor condensation system comprise the dryer. Maintaining the pressure in the drying chamber below the water vapor pressure of the ice surface achieves continuous removal of water vapor from the product. A vacuum pump removes the water vapor. The pressure reduction immediately after the product enters the vacuum chamber should be carried out very quickly, along with low pressure in the vacuum chamber and the constant maintenance of the pressure during dehydration, otherwise, ice melting occurs and water forms.
In the third and final stage, heat is supplied to the product which is equal to the latent heat of sublimation of ice, i.e., it cannot be higher than the freezing point of the product and hence, the nutritional value of the product is not degraded. The required heat is supplied to the food via conduction, radiation, or a combination of the two methods, or even by the application of microwaves. In any case, the water vapor produced during the sublimation of the ice crystals is removed through the porous system created by the sublimation in the food, and then, the condenser of the dryer removes it before reaching the vacuum pump.
Finally, although the freeze-drying technique is successful for almost all food ingredients (with the exception of those that are difficult to dry), its cost seems to be five times the cost of spray drying. The encapsulation of water-soluble spirits, natural perfumes, and medicines is accomplished by this technique. Examples of applications are protein powders, lyophilized curcumin powder, etc., where their redispersion in water activates the activity of bioactives [21].
Freeze drying (FD) is the most common method for drying heat-sensitive ingredients in the food and pharmaceutical industries, maintaining a number of viable probiotics. The probiotic suspension freezes below its eutectic point, while the removal of the frozen water is achieved by the low pressure effect. The cell membrane is being damaged by the formation of ice crystals during the freeze-drying process. The cell membrane and cell proteins are stabilized by cryoprotectants such as lactose, trehalose, maltodextrin, sorbitol, sucrose, or milk proteins. Freeze-dried probiotics should remain active during storage. Storage conditions therefore play an important role in their viability, which generally appears high at temperatures < 4 °C and low at temperatures between 20 and 30 °C [7].
Freeze drying stabilizes probiotic bacteria after drying and storage, and more specifically, this technique stabilized L. rhamnosus, as reported by Moayyedi et al. [14], L. acidophilus [22], Lactococcus lactis [23], L. plantarum, and L. acidophilus [24]. Shu et al. [22] observed that L. acidophilus survival improved following the addition of sodium phosphate in the cryoprotective medium. A high viability is mentioned at conditions below 4 °C, whereas environmental temperatures (approximately 20 to 30 °C) commonly reduce probiotic viability. Moreover, encapsulation agents, probiotic strain, and storage conditions, such as water activity (RH) and temperature, might enhance probiotic viability, as reported by all of these studies mentioned above. Amounts between 7–~8 logs CFU/g of these probiotics have been discussed after exposure to simulated GI fluids.
4. Fluidized Bed Coating
Fluidized bed coating is used in the encapsulation of mainly sensitive components, in which spraying an encapsulating agent into a fluidized bed of powder produces coated particles. It is often combined with spray drying technology.
More specifically, a uniform layer is formed around the particles as the coating agent is deposited on the surface, thus enhancing the barrier properties and increasing the protection of sensitive components [25][26]. An air stream suspends the dust particles at a precise temperature and these are then sprayed with a coating material. Aqueous solutions of gums, starch, cellulose, and proteins could be coating materials, and these materials should have an acceptable viscosity, be thermally stable, and be able to form a film around the particle. The fluidized bed coating process involves three main steps: (a) the powder particles to be coated in the coating chamber need to be air stream fluidized, (b) the nozzle sprays the coating material onto the particles, and (c) the coating material sticks to the particles via evaporation of the coating material solvent with hot air. The most important parameters with an impact on agglomeration and particle formation affecting coating performance are humidity, spray pressure, coating feed rate, and temperature [27]. Fluidized bed coating is used in the nutritional supplement market to deliver encapsulated nutrients such as a variety of vitamins and minerals. In the meat industry, many acids have been encapsulated by this technique to improve color and flavor, as well as to reduce processing time. It is also used in bakery products to coat additives such as acetic acid, lactic acid, sorbic acid, potassium sorbate, calcium propionate, and salt [26].
High cost and direct exposure to high temperature, which can cause particle degradation and possible particle agglomeration, are some of the disadvantages of fluidized bed coating. Introducing more process parameters that could potentially affect the product properties makes the process much more complicated. However, the flexibility of this technique could easily be adopted for the mass production of biospheres, hence its use in the production of commercial dried yeast [7][28].
For probiotic encapsulation, a previous treatment of the cells is required to promote a solid particle which can be suspended and covered, characterizing the fluidized bed as a co-encapsulation technique [29][30][31]. Cellulose has been employed as an encapsulating material to encapsulate Enterococcus faecium IFANo.045 and L. plantarum IFANo.278 for the use of the fluidized bed technique, as reported by Strasser et al. [32]. They also emphasized that adding sucrose and trehalose to wall materials increased cell protection rates. Fluidized bed coating was also effective in producing alginate–chitosan microcapsules containing L. plantarum NCIMB 8826. This improved the storage cell survival of encapsulated probiotics compared to free cells, as reported by Albadran et al. [33].
5. Extrusion
Extrusion being a simple, low-cost encapsulation technology does not use harmful solvents, with efficient operation under mild conditions without advanced equipment [34][35]. It can be considered as an ideal technique for the encapsulation of thermosensitive bioactives, such as probiotics and ω-3 fatty acids. More specifically, this technique involves mixing bioactives into a hydrocolloid solution which is then extruded through a nozzle into a curing solution (e.g., CaCl2, AlCl3, or FeCl2). Thus, the initial mixture turns into a gel and pellets are created. The diameter of the nozzle, the distance between the nozzle, and the curing solution, as well as the concentration of the hydrocolloid solution, affect the size of the beads produced [3][36]. Various polysaccharides (most commonly sodium alginate) can be used for extrusion encapsulation, but the choice of these will determine the size, shape, and viability of the encapsulated material [37].
Extrusion is distinguished based on the operating method of the extruder, either hot or cold. Hot extrusion uses high pressures and temperatures. The rapid release of pressure as the food exits the dye causes the steam and gases to instantly expand through the product, forming a low-density food. Thus, a variety of different shapes such as spheres, tubes, sticks, etc., are obtained. In cold extrusion, the temperature of the food remains the same as that of the environment. In this case, the screw rotates at a low speed without developing friction and significant pressures, therefore the product does not undergo changes in its structure.
Hot and cold extrusion can be employed as a preservation method in order to reduce the water activity of the food. The main disadvantage of this technique used by the food industry is the formation of large particles and a slow production rate. This can be compensated for by modifying the technique with electrostatic air flow extrusion, rotating disk spraying, or using multiple nozzles simultaneously [3].
Haghshenas et al. [38] encapsulated L. plantarum 15HN using alginate, an alginate–psyllium blend, and alginate–fenugreek blend. To produce the beads, the solutions were extruded through a 21-gauge nozzle in a sterile calcium chloride solution. Etchepare et al. [39] used resistant starch, chitosan, and alginate to encapsulate L. acidophilus La-14 via extrusion in a calcium chloride solution using an aerograph coupled to an air compressor. Rodrigues et al. [35] used alginate combined with natural polysaccharides present in linseed and okra mucilages, botryosphaeran (exopolysaccharide produced by the endophytic fungus, Botryosphaeria rhodina MAMB-05), and fructo-oligosaccharides to encapsulate strains of Lactobacillus casei 01 and BGP 93 via extrusion. A protective effect on the cell viability of the encapsulated probiotic during 15 days of refrigerated storage was observed.
6. Ionic Gelation
An electrostatic interaction between opposite charges containing at least one polymer carries out encapsulation using this technique. The ionic gelation method is most often used to prepare alginate particles. Initially, an aqueous polymer solution containing low molecular mass ions (e.g., CaCl2, BaCl2, MgCl2, CuCl2, or ZnCl2) which react with oppositely charged electrolytes, resulting in an insoluble gel, starts ionic gelation. This mixture is added dropwise to a solution containing oppositely charged ions under vigorous and constant stirring. The formation of spherical particles due to the complexation between the oppositely charged ions is the initiation of ionic gelation. The spheres are then removed via filtration, poured with distilled water, and dried. It is a simple, cheap, and fast method with no requirement for special equipment, high temperatures, or organic solvents. However, it is considered to be disadvantageous due to the difficulty in producing uniform-sized particles [17][40].
The type and concentration of polymers used in ionic gelation determine the success of ionic gels [28]. Alginate particles (AMPs) were used for the encapsulation of B. licheniformis BCR 4–3 marine probiotics for improved storage stability and targeted delivery within shrimp intestines. The AMPs were able to effectively deliver probiotic bacteria that remained effective and stable [41]. The viability (from 3.28 log to 3.23 log CFU/mL) of the L. acidophilus (1643 PtCC) in a biliary salt condition for 120 min was sustained by Ebrahimnejad et al. [42] when they used chitosan and tripolyphosphate anions as wall materials.
The use of chitosan as a coating material on alginate beads containing the bacterium Bifidobacterium longum has been shown to improve its survival in gastric fluid and high-temperature conditions. However, the disadvantage of its use as an encapsulation material for probiotics is the inhibitory effect that it exhibits against microorganisms, including lactic acid bacteria [36].
Vaziri et al. [43] reported that the use of sodium alginate and alginate–chitosan blends crosslinked with Na-tripolyphosphate enhanced the encapsulation efficiency of L. plantarum from 97 to 100%. Different probiotics have been encapsulated via the ionic gelation process such as L. casei, L. rhamnosus, L. plantarum, Pediococcus pentosaceus, L. fermentum, and L. acidophilus [44][45][46][47][48][49]. Regarding the survival rate, it has been reported that it depends on the sensitivity of the probiotic strain. In a study by Rather et al. [50], L. plantarum had a survival rate higher than L. casei. Double crosslinking using Na-TPP improved the viability of L. plantarum (93%) compared with simple alginate crosslinking alone (85%), as shown by Vaziri et al. [45].
7. Complex Coacervation
Microencapsulation through coacervation is carried out in three stages via continuous stirring. In the first stage, the formation of three chemically immiscible parts takes place: the core medium, the assembly fluid, and the coating substance. In the second stage, the base substance is diffused into the coating solution. In the third stage, the outer layer is solidified via chemical or physical crosslinking reactions [7][51]. It is a low-cost technique which has a high loading capacity, has no release difficulties, does not require the use of organic solvents, and does not require the presence of extreme reaction conditions to harden the core shell. This process uses gum arabic (GA), chitosan, mesquite gum, pectin, alginate, xanthan gum, carrageenan, and carboxymethyl cellulose from polysaccharides, gelatin, whey protein, casein, albumin, and plant proteins such as pea or soy protein [7].
Yin et al. [52] encapsulated probiotics Lactobacillus rhamnosus GG in coacervates formed by whey protein isolate-high melting point fat shortening oil (SO) and gum arabic through complex coacervation and reported a higher survival rate of 54.96%. Complex coacervation leads to the formation of stable and strong network coacervates around the core materials, following the formation of the two-phase system based on the electrostatic attraction of two polyelectrolytes with opposite charges [53][54].
Different probiotics have been encapsulated via the complex coacervation process such as L. plantarum, casei, paracasei, B. lactis, L. acidophilus, and L. reuteri [55][56][57][58][59][60]. Regarding viability, Bosnea et al. [57] found that the encapsulation of probiotics in WPI:GA coacervates grew slightly faster than free bacteria under simulated gastric conditions, whereas Mao et al. [58] reported that the preservation of B. longum under simulated GI conditions was accomplished via soy protein microcapsules isolated from i-carrageenan (ratio of 10:1).
8. Electrospray and Electrospinning as Part of Nanoencapsulation
Nanoencapsulation is a strategy ultimately leading to the entrapment of a core component into a protective nanocarrier. Several strategies can be employed to carry out the process. Antisolvent precipitation, electrospinning, electrospraying, the emulsion–evaporation method, high-pressure microfluidization, ionic gelation, lyophilization, nanoemulsification, nano spray drying, and ultrasonication-assisted emulsification are some of the technologies used in the production of nano-encapsulated materials [61][62][63][64]. Many factors such as the core component and wall material properties, as well as the interaction between them and the intended application, might affect the selection of the method.
Nanoencapsulation in the context of sustainable food production is described in Table 1, for example, the incorporation of hydrophobic compounds in foods due to reduced miscibility among food components.
Table 1.
Nanoencapsulation of probiotics and their applications in sustainable food production.
| Encapsulation Process | Probiotics | Wall Material(s) | Effect | Reference |
|---|---|---|---|---|
| Νanocoating | Lactobacillus acidophilus | Artificial nanoshells | Enhanced viability of nanocoating L. acidophilus in simulated gastric fluid (SGF) by 49% compared with free probiotics. | [65] |
| Electrospinning | Probiotic strains of LAB and Bifidobacteria | Corn starch (CS) and sodium alginate (SA) nanofiber | After a 20-day storage of yogurt, the count of nanoencapsulated LAB and bifidobacteria declined by 0.19 (97.9% survival) and 0.28 (96.9% survival) log CFU, respectively. | [66] |
| Electrospinning | Probiotic strains | Starch/sodium alginate | Enhanced viability rate of lactobacilli and bifidobacteria strains in the acidic environment and simulated gastrointestinal conditions. | [67] |
| Whey protein isolate fibrils (WPIFs), sodium alginate (ALG), carboxymethyl cellulose (CMC), and xanthan gum (XG) | Enhanced survival of | Lactobacillus plantarum | 90 (LP90). | [68] |
The use of gelatin nanofibers for the improvement in the solubilization of lycopene was explored by Horuz and Belibağlı [69] who found a significant increase in the solubility in aqueous solution by encapsulating it with gelatin nanofibers.
A similar approach was explored in a study with cheese crackers [70]. The nanoparticles of sodium chloride were produced by nano spray drying and applied to the surface of samples (1, 1.5, and 2% of sodium chloride). Higher scores for saltness than the control (2% of sodium chloride) were received in fresh samples. Higher scores in saltness were reported in samples produced with nanoparticles compared to the control after 4 months of storage. A similar experiment was carried out with potato chips by Vinitha et al. [71], where the sodium chloride nanoparticles were generated via an electrohydrodynamic atomized drying method. The perception of saltness in relation to the control chips with commercial size sodium chloride was increased by the use of nanoparticles, and sodium chloride was reduced by 65% [72].
During the nanoencapsulation of probiotics, a protective nanoshell is formed that aims to surround them. Nanocapsules, nanoemulsions, nanoliposomes, and nanoparticles are the most common formations. Nanocellulose, starch nanoparticles (SNPS), titanium dioxide, silicon dioxide, and zinc oxide nanoparticles are also the most commonly used nanomaterials. The nanoscale coating limits the loss of integrity of the probiotics until they reach the gut while still managing to transport and reach their target under adverse conditions, as reported by authors in Table 1.
Electrospraying and electrospinning are electrohydrodynamic (EHD) techniques, characterized as simple flexible and mild techniques managing to attract interest for encapsulating and immobilizing probiotics. From these emerging techniques, nano/microscale fibers and spheres are produced [73]. A needle using a syringe pump carries out the extrusion of the polymer solution. The formation of a Taylor cone at the tip of the needle takes place following the emergence of the droplet. Explosion of the jets out of the cone then occurs, followed by collection of the broken droplets by the collector [74].
Xu et al. [75] reported an increased survival rate of 89.26% after electrospinning and an increased survival rate of 84.63% after 21 days of storage of Lactobacillus rhamnosus 1.0320 when they used poly(vinyl alcohol) (PVA)/pectin (PEC) as the wall material. Survivability of 90.07% and 91.96% in simulated gastric and intestinal fluids of Lactobacillus rhamnosus 1.0320 was maintained by Xu et al. [76] when they used PVA/PEC. Ma et al. [77] managed to enhance the stability of cells of L. plantarum KLDS 1.0328 and GA when they used PVA as the wall material.
9. Emulsification
Emulsification is commonly used to encapsulate lipid bioactives. The dispersion of the encapsulated material (discontinuous phase) in an organic phase (continuous phase) takes place here via the formation of an oil-in-water emulsion. The emulsion is prepared by homogenizing the mixture with the help of surfactants [40][78]. Often, it is used as a preparatory step for other techniques, e.g., ionic gelation, spray drying, etc.
10. Encapsulation in Yeasts
The application of yeast cells as encapsulation agents has become evident in recent years. The most obvious benefit of this application is that the cell itself takes the shape of an oval capsule that can be used as it is. The thick (100–200 nm) and β-glucan-rich cell wall as well as the plasma membrane provides the robust structure, thus holding both hydrophilic and hydrophobic compounds within its bulk. The diffusion of compounds according to their molecular weight and hydrophobicity are allowed due to the porosity of the cell wall, and this provides good mechanical strength. In addition, yeasts are easier to cultivate on an industrial scale [16]. Incubation of the cells with the material to be encapsulated forms a simple impregnation process, and this has been described by Dimopoulos et al. [79], showing benefits of the encapsulation of compounds inside the cell. They encapsulated the essential oil of oregano in cells of Saccharomyces cerevisiae in order to cover its strong smell but also to protect its bioactives from external processing parameters.
A wide range of applications, such as food, medicine, or cosmetics, use yeast cell capsules. Yeast cells are not empty in their natural state. Cytosol removal can be carried out via extraction, hence the flavor of the yeast itself is reduced and more space is provided for the encapsulation of valuable substances. The cell wall is the way for substances to be encapsulated in yeast cells since they must pass through that to enter the cells [80]. This can be achieved in the following ways [79]:
-
Autolysis: The permeability of yeast cells can be increased by this method or cells can be broken with the aim of obtaining intracellular products. When the cell’s own enzymes proceed to hydrolyze its macromolecules, this is called yeast autolysis. The cell wall structure is affected only in terms of permeability and retains its shape after the procedure is completed, as evidenced by electron microscopy. The main damage observed is located in the plasma membrane. In the food industry, autolysis takes place under conditions of temperatures of approx. 50 °C and pH = 5.5.
-
Pulsed electric fields: The membrane of the yeast cells is exposed to an external high-voltage electric field, thus resulting in the formation of pores on the surface of the membrane, and this is called pulsed electric field treatment. Moderate electric field strengths of yeast cells do not cause cell disruption but lead to increased cell permeability at both the cell membrane and cell wall level. However, damage to the cell such as gaps in the cell wall and shrinkage of the cytoplasm is caused at very high electric field strengths (40 kV/cm). This technique has been studied in yeast cells with the aim of increasing the extraction of yeast extract, polysaccharides, proteins, and enzymes.
-
High-pressure homogenization: It is a method to break up cells in large-scale processing. Intracellular products such as proteins from E. coli and S. cerevisiae can be obtained by this method. Being a non-thermal method, the pressure of the cell suspension is increased up to a pressure of 1000 bar, hence a specially designed valve system of the homogenizer releases the suspension. Cells receive a wide range of forces that can even cause their complete lysis and, by extension, an increase in cell permeability.
-
Plasmolysis: plasmolysis involves cell incubation in the presence of NaCl or ethyl acetate, causing the cell membrane to rupture due to osmotic stress.
The conventional process for encapsulation can be divided into three main steps: mixing of the yeast and bioactives in water at a certain period allowing the substance to migrate into the cells, removal of any unencapsulated bioactives in order to wash the cells, and spraying or lyophilization so that the drying of the cells containing bioactives is accomplished, hence acquiring the binding of the substances inside the cells.
The cell wall pores close again and the substances remain trapped inside them when the yeast cells are dried [80].
The immobilization of yeast cells is currently used in industrial winemaking (i.e., sparkling wine and fino sherry wine) and brewing. The production of sparkling wine has been accomplished by yeast cells encapsulated in alginate beads, as reported by [81][82][83].
Until now, the production of fermentation beverages such as white wine, sparkling wine, natural sweet wine, beer, red raspberry wine, and bioethanol from molasses or starch has been achieved by the use of biocapsules [84][85][86][87][88][89][90][91][92][93].
However, a new methodology to assemble biocapsules for the infusion and immobilization of yeast cells into filamentous fungal pellets, which serve as a porous natural material, has been reported to cover the restriction of the microorganisms’ growth [94]. Yeast cells can be forced towards the core of the fungal pellets followed by culture in a YPD medium to promote their growth from the interior via vacuum application.
This technique involves four steps: (i) separation of yeast cells and culture filamentous fungal spores to promote biomass production and avoid microorganism competition, (ii) increase yeast cell population in the pellet core by mixing the resulting pellets and yeasts suspension, and yeast cell infusion into the pellets through a vacuum, (iii) YPD liquid medium culturing to promote further attachment of the yeast and pellet, and (iv) removal of non-attached cells in the pellet surface by washing. The biocapsules formed have been named “microbial biocapsules’’.
11. Liposomes
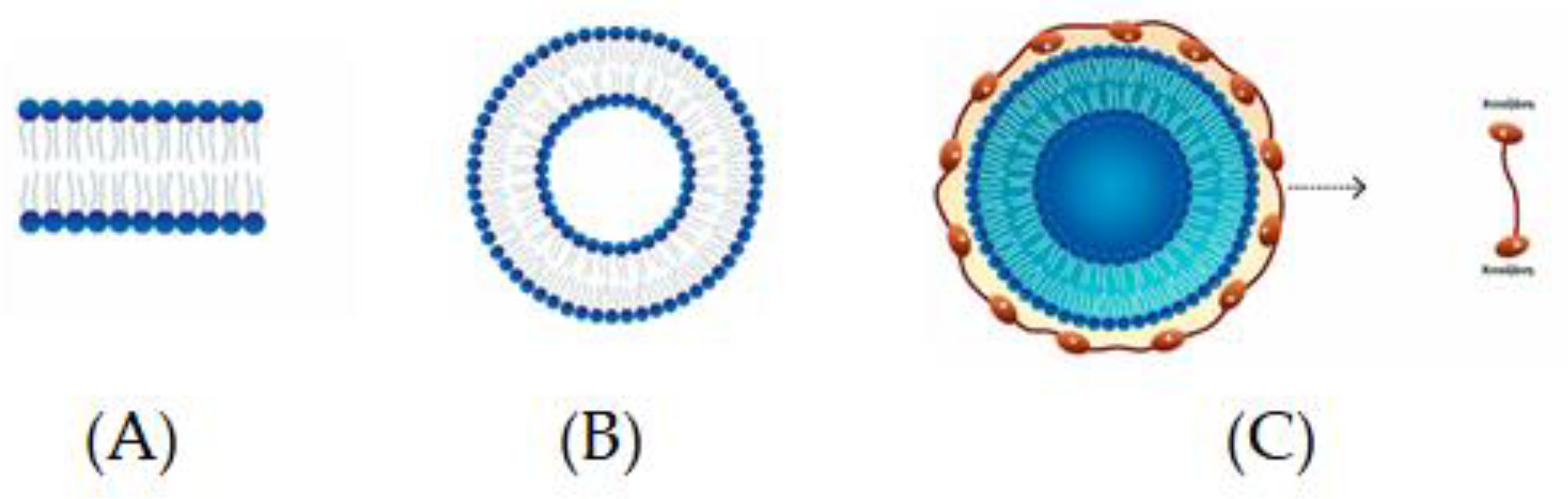
Spherical structures composed of bilayers of phospholipids, which are distinguished by a polar head or hydrophilic unit and two hydrocarbon hydrophobic tails, are called liposomes (Figure 1A,B) [95]. The encapsulation of food components of different polarity, so that a natural barrier is formed and bioactives are protected from external conditions, can be carried out via the presence of liposomes due to their amphiphilic nature and their simultaneous occurrence [95]. Some of the distinct advantages of liposomes in encapsulation are as follows: (a) better physical stability, (b) controllable particle size, (c) ease of preparation and the possibility of industrial-scale production, (d) the encapsulation of both lipophilic and hydrophilic components, (e) the controlled and sustained release of the ingredients, (f) the non-use of organic solvents, and (g) the cost-effectiveness [96].
The thermodynamic instability and the disruption of their structure during food processing, causing disintegration and release of entrapped compounds, are some of the limitations of liposomes. Hence, the evaluation of the behavior of the particles over time under different conditions such as pH and temperature is being studied by stability studies. Controlled release of the encapsulated ingredients and in vitro studies are conducted to test the release of these ingredients in GIT conditions in stability studies. Many authors propose the application of biopolymers as coating materials such as chitosan to be widely used for coating liposomes to modify the surface of liposomes in order to make them more protected, stable, and consequently applicable. Examples of other biopolymers that are also frequently used are starch, alginate, whey protein, and gelatin [95]. The coating of liposomes with biopolymers, such as polysaccharides and proteins, is well known as surface nanoengineering (modification of the surface). Protective films such as food-grade natural biodegradable polymers are used as a novel approach to nanoencapsulation in the food manufacturing sector [97]. In this direction, gelatin (GE)–chitosan (CH) polyelectrolyte-coated nanoliposomes were developed and characterized by Adeel et al. [98] to prolong the viability of probiotics (L. acidophilus). Hence, these nanoliposomes could be used as an effective carrier for the delivery of probiotics.
Chitosomes
A novel way to improve the encapsulation efficiency of liposomes is to modify the surface of the liposomal membrane through the formation of bioadhesive and polymeric layers. Chitosan is a linear natural polysaccharide widely applied in biomedical and biotechnological fields due to its biocompatibility, bioadhesiveness, biodegradability, and non-toxicity. Hence, the development of phospholipid–chitosan composite vesicles (chitosomes) as novel structures for the encapsulation of drugs and nutrients is of interest [99] (Figure 1C).
Chitosan can be obtained via partial deacetylation of chitin. In an aqueous solution with a pH < 6.5, the amines of chitosan are protonated. They can be applied as a coating material due to the electrostatic attraction between the protonated amino groups of chitosan and the negatively charged groups present on the surface of liposomes. Chitosomes form thick layers around liposomes with the immediate result being an increase in particle size. For chitosomes preparation, it is usually recommended to produce liposomes normally and then promote the electrostatic deposition of cationic chitosan on the liposome surface via the addition of a chitosan solution under stirring [95]. Alginate–chitosan has been used for the encapsulation of Lactobacillus rhamnosus ASCC 290 and L. casei ATCC 334, as reported by Farias et al. [100]. This provided an encapsulation efficiency of >76%, and both bacteria were protected under simulated gastrointestinal conditions.
12. Inclusion Complexation
This method is performed with cyclodextrins (CDs) as encapsulating materials. CDs are cyclic oligosaccharides derived from starch via enzymatic cleavage. α-, β-, and γ-CDs have 6, 7, and 8 glucose units, respectively, covalently attached. Their characteristic is the hydrophobic inner cavity and the hydrophilic outer surface [16]. Structural differences make the physicochemical properties of α-, β-, and γ-CDs differ slightly. A relatively small molecular cavity, so it can only accept small molecules, makes α-CD research limited. The larger molecular cavity but high production cost and its non-suitability for large quantities are characteristics of γ-CD. β-CD is simple and economical to produce on a large scale, with a reasonable cavity size, and is applied to commercialized foods.
The only encapsulation method that occurs at the molecular level is CD encapsulation. CD can neutralize characteristic odors, e.g., of garlic and onion oils, complexing their volatile components. The formation of complexes with CD allows the controlled release of bioactives and may prove useful for shelf-life optimization and the bioavailability of encapsulated compounds. It has also been reported that CD enhances the stability of fat-soluble vitamins A, E, and K [17]. Finally, there are many encapsulation methods using cyclodextrins such as spray drying and lyophilization.
13. Hydrogels
Protein–polysaccharide composites have constructed hydrogel systems, and this has attracted researchers’ attention due to their biodegradability, biocompatibility, and non-toxic properties. Soy protein isolate–citrus pectin composite hydrogels induced by transglutaminase (TGase) and ultrasonic treatment for 20 min protected the survival of Lactiplantibacillus plantarum (new name of L. Plantarum) from gastrointestinal digestion and UV irradiation, as reported by Liu et al. [101]. Moreover, Zhang et al. [102] constructed double-saccharide composite hydrogel beads for the encapsulation of Companilactobacillus crustorum MN047 by applying different combination ratios of low methoxyl pectin (LMP) and sodium alginate (SAG) and the concentrations of calcium chloride (CaCl2).
In Table 2, the advantages and disadvantages of each encapsulation technique are described.
Table 2.
Advantages and disadvantages of encapsulation techniques of probiotics.
| Advantages | Disadvantages | References | ||
|---|---|---|---|---|
| Spray drying | ||||
| Short drying time; Low cost; Flexibility; High productivity process; Large-scale production; High speed. |
Decreased number of viable probiotic organisms; Wall material must be soluble in water; Particles with irregular geometry and porous surface; Process losses of heat-sensitive substances have not been fully addressed. |
[1][6][7][15][28][41][71] | ||
| Spray cooling | Layer-By-Layer Coating | Lactobacillus plantarum 90 (LP90) | ||
| Low-cost, fast process; Dense, spherical, and smooth particle surface; It can be applied on an industrial scale. |
Low performance; Not suitable for heat-sensitive bioactives; Possibility of loss of bioactives. |
[15][17] | ||
| Freeze drying or lyophilization | ||||
| Mild conditions; Suitable for heat-sensitive compounds; Almost unchanged nutritional value; Rapid comprehensive dehydration. |
Decreased number of viable probiotic organisms; High cost of installation and operation of the equipment; Unsuccessful on foods that are difficult to dehydrate; Risk of cell damage. |
[21][71] | ||
| Fluidized bed coating | ||||
| Suitable for thermally sensitive components; Mass production of biospheres. |
Complicated process; Direct exposure to high temperature can cause molecules to degrade; High cost. |
[7][25][28] | ||
| Extrusion | ||||
| Simple, low-cost technology; Mild conditions; Ideal for thermosensitive bioactives. |
Reduced viability after the extrusion process; Formation of large particles; Slow production rate; Not applicable on an industrial scale; The use of hydrocolloid solution is necessary. |
[3][15][28] | ||
| Ionic gelation | ||||
| Simple and economical method; No special equipment or organic solvents are required; Mild conditions. |
It is not easy to produce particles of uniform size; | [17][36][40][42] | ||
| Emulsification | ||||
| Easy to scale up; Simple and flexible technique; High sustainability of bioactives, e.g., probiotics. |
Not suitable for mass production; Non-uniform particle shape and size. |
[28][41][71][103] | ||
| Encapsulation in yeasts | ||||
| Simple and low-cost process; High encapsulation efficiency; Good mechanical strength and easy cultivation on an industrial scale; No additional materials are used. |
Mainly used for the encapsulation of lipophilic molecules. | [16] | ||
| Liposomes | ||||
| Encapsulation of hydrophilic and hydrophobic molecules; Production of uniform pellets on an industrial scale; No use of organic solvents. |
Thermodynamically unstable method; The structure of the liposomes can be disrupted and the encapsulated substances can be released. |
[95][96] | ||
| Inclusion complexation | ||||
| Economical to produce it on a large scale (β-cyclodextrin); Also suitable for volatile compounds; It allows the controlled release of bioactives. |
High doses of cyclodextrin may be harmful. | [16][17] | ||
References
- Desobry, S.; Gaiani, C.; Jayaprakash, P.; Maudhuit, A. Encapsulation of bioactive compounds using competitive emerging techniques: Electrospraying, nano spray drying, and electrostatic spray drying. J. Food Eng. 2023, 339, 111260.
- Abdul-Mudalip, S.K.; Arshad, Z.I.; Che-Man, R.; Hashim, N.A.; Khatiman, M.N. A short review on encapsulation of bioactive compounds using different drying techniques. Mater. Today Proc. 2021, 42, 288–296.
- Alvarez-Salas, C.; Espinosa-Solis, V.; Leyva-Porras, C.; Piñón-Balderrama, C.; Saavedra-Leos, M.Z.; Terán-Figueroa, Y. Encapsulation of active ingredients in food industry by spray-drying and nano spray-drying technologies. Processes 2020, 8, 889.
- Akbari-Alavijeh, S.; Boostani, S.; Dima, C.; Falsafi, S.R.; Geranpour, M.; Hosseini, H.; Jafari, S.M.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Samborska, K.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325.
- Zhu, Q.; Tang, J.; Yao, S.; Feng, J.; Mi, B.; Zhu, W.; Chen, Q.; Liu, D.; Xu, E. Controllable structure of porous starch facilitates bioactive encapsulation by mild gelatinization. Food Hydrocoll. 2023, 145, 109135.
- Arepally, D.; Goswami, T.K. Effect of Inlet Air Temperature and Gum Arabic Concentration on Encapsulation of Probiotics by Spray Drying. LWT-Food Sci. Technol. 2019, 99, 583–593.
- Barajas-Álvarez, P.; González-Ávila, M.; Espinosa-Andrews, H. Recent Advances in Probiotic Encapsulation to Improve Viability under Storage and Gastrointestinal Conditions and Their Impact on Functional Food Formulation. Food Rev. Int. 2023, 39, 992–1013.
- Gonzalez-Ferrero, C.; Irache, J.M.; Gonzalez-Navarro, C.J. Soybean Protein-Based Microparticles for Oral Delivery of Probiotics with Improved Stability during Storage and Gut Resistance. Food Chem. 2018, 239, 879–888.
- Nunes, G.L.; Etchepare, M.D.A.; Cichoski, A.J.; Zepka, L.Q.; Jacob Lopes, E.; Barin, J.S.; Flores, E.M.D.M.; Da Silva, C.D.B.; De Menezes, C.R. Inulin, Hi-Maize, and Trehalose as Thermal Protectants for Increasing Viability of Lactobacillus acidophilus Encapsulated by Spray Drying. LWT-Food Sci. Technol. 2018, 89, 128–133.
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with Fructooligosaccharide as Wall Material by Spray Drying. LWT-Food Sci. Technol. 2015, 60, 773–780.
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by Spray Drying Related to Its Stability and In Vitro Digestion. LWT-Food Sci. Technol. 2017, 82, 82–89.
- Tantratian, S.; Wattanaprasert, S.; Suknaisilp, S. Effect of Partial Substitution of Milk-Non-Fat with Xanthan Gum on Encapsulation of a Probiotic Lactobacillus. J. Food Process. Preserv. 2018, 42, e13673.
- Loyeau, P.A.; Spotti, M.J.; Vanden Braber, N.L.; Rossi, Y.E.; Montenegro, M.A.; Vinderola, G.; Carrara, C.R. Microencapsulation of Bifidobacterium Animalis Subsp. Lactis INL1 Using Whey Proteins and Dextrans Conjugates as Wall Materials. Food Hydrocoll. 2018, 85, 129–135.
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.T. Effect of Drying Methods (Electrospraying, Freeze Drying and Spray Drying) on Survival and Viability of Microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods. 2018, 40, 391–399.
- Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielińska, A.; Souto, E.B. Encapsulation of active pharmaceutical ingredients in lipid micro/nanoparticles for oral administration by spray-cooling. Pharmaceutics 2021, 13, 1186.
- Duary, R.K.; Kumar, A.; Mahato, D.K.; Pandhi, S.; Premjit, Y.; Rai, D.C. Current trends in flavor encapsulation: A comprehensive review of emerging encapsulation techniques, flavor release and mathematical modelling. Food Res. Int. 2022, 151, 110879.
- Alhamad, M.; Alrosan, M.; Aludatt, M.H.; Alzoubi, H.; Alzougl, R.; Gammoh, S.; Ghatasheh, S.; Ghozlan, K.; Rababah, T.; Tan, T.C.; et al. Encapsulation-based technologies for bioactive compounds and their application in the food industry: A roadmap for food-derived functional and healthy ingredients. Food Biosci. 2022, 50, 101971.
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT-Food Sci. Technol. 2017, 81, 160–169.
- Arslan-Tontul, S.; Erbas, M.; Gorgulu, A. The use of probiotic-loaded single-and double-layered microcapsules in cake production. Probiotics Antimicrob. Proteins 2019, 11, 840–849.
- Bampi, G.B.; Backes, G.T.; Cansian, R.L.; de Matos, F.E.; Ansolin, I.M.A.; Poleto, B.C.; Corezzolla, L.R.; Favaro-Trindade, C.S. Spray chilling microencapsulation of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis and its use in the preparation of savory probiotic cereal bars. Food Bioproc. Technol. 2016, 9, 1422–1428.
- Dong, H.; Li, R.; Shen, J.; Wang, P.; Xu, X.; Yang, Z. Dual improvement in curcumin encapsulation efficiency and lyophilized complex dispersibility through ultrasound regulation of curcumin-protein assembly. Ultrason. Sonochem. 2022, 90, 106188.
- Shu, G.; Wang, Z.; Chen, L.; Wan, H.; Chen, H. Characterization of Freeze-Dried Lactobacillus Acidophilus in Goat Milk Powder and Tablet: Optimization of the Composite Cryoprotectants and Evaluation of Storage Stability at Different Temperature. LWT-Food Sci. Technol. 2018, 90, 70–76.
- Archacka, M.; Białas, W.; Dembczyński, R.; Olejnik, A.; Sip, A.; Szymanowska, D.; Celińska, E.; Jankowski, T.; Olejnik, A.; Rogodzińska, M. Method of Preservation and Type of Protective Agent Strongly Influence Probiotic Properties of Lactococcus lactis: A Complete Process of Probiotic Preparation Manufacture and Use. Food Chem. 2019, 274, 733–742.
- Da Silva Guedes, J.; Pimentel, T.C.; Diniz-Silva, H.T.; Da Cruz Almeida, E.T.; Tavares, J.F.; Leite De Souza, E.; Garcia, E.F.; Magnani, M. Protective Effects of β-Glucan Extracted from Spent Brewer Yeast during Freeze-Drying, Storage and Exposure to Simulated Gastrointestinal Conditions of Probiotic Lactobacilli. LWT-Food Sci. Technol. 2019, 116, 108496.
- Cloutier, S.; Nickerson, M.; Yan, C.; Zhang, W. Protection and masking of omega-3 and -6 oils via microencapsulation. In Microencapsulation in the Food Industry; Academic Press: Cambridge, MA, USA, 2014; pp. 485–500.
- Dewettinck, K.; Huyghebaert, A. Fluidized bed coating in food technology. Trends Food Sci. Technol. 1999, 10, 163–168.
- Reddy, C.K.; Agarwal, R.K.; Shah, M.A.; Suriya, M. Encapsulation Techniques for Plant Xxtracts in Plant Extracts: Applications in Food Industry; Academic Press: Cambridge, MA, USA, 2022; pp. 75–88.
- Rajam, R.; Subramanian, P. Encapsulation of probiotics: Past, present and future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46.
- Manojlović, V.; Nedović, V.A.; Kailasapathy, K.; Zuidam, N.J. Encapsulation of probiotics for use in food products. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer Science+Business Media: New York, NY, USA, 2010; pp. 269–302.
- Chávarri, M.; Maranon, I.; Villaran, M.C. Encapsulation technology to protect probiotic bacteria. In Probiotics; Rigobelo, E.C., Ed.; InTech: London, UK, 2012; pp. 501–540.
- Ozdal, T.; Yolci-Omeroglu, P.; Tamer, E.C. Role of encapsulation in functional beverages. In Biotechnological Progressand Beverage Consumption; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing, Elsevier: Cambridige, MA, USA, 2020; Volume 19, pp. 195–232.
- Strasser, S.; Neureiter, M.; Geppl, M.; Braun, R.; Danner, H. Influence of lyophilization, fluidized bed drying, addition of protectants, and storage on the viability of lactic acid bacteria. J. Appl. Microbiol. 2009, 107, 167–177.
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216.
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13.
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.; Barbosa-Dekker, A.M.; Garcia, S. Effect of natural polymers on the survival of Lactobacillus casei encapsulated in alginate microspheres. J. Microencapsul. 2017, 34, 431–439.
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications–A narrative review. Food Res. Int. 2020, 137, 109682.
- Giannou, V.; Frakolaki, G.; Topakas, E.; Tzia, C. Effect of various encapsulating agents on the beads morphology and the viability of cells during BB-12 encapsulation through extrusion. J. Food Eng. 2021, 294, 110423.
- Haghshenas, B.; Abdullah, N.; Nami, Y.; Radiah, D.; Rosli, R.; Yari Khosroushahi, A. Microencapsulation of probiotic bacteria Lactobacillus plantarum 15 HN using alginate-psyllium-fenugreek polymeric blends. J. Appl. Microbiol. 2015, 118, 1048–1057.
- Etchepare, M.; Raddatz, G.C.; Flores, E.M.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Raimundo, C.; Grosso, F.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT-Food Sci. Technol. 2016, 65, 511–517.
- Buensanteai, N.; Huy Hoang, N.; Kamkaew, A.; Le Thanh, T.; Papathoti, N.K.; Saengchan, C.; Sangpueak, R.; Thepbandit, W.; Treekoon, J. Chitosan nanoparticles-based ionic galation method: A promising candidate for plant disease management. Polymers 2022, 14, 662.
- Vega-Carranza, A.S.; Cervantes-Chávez, J.A.; Luna-Bárcenas, G.; Luna-González, A.; Diarte-Plata, G.; Nava-Mendoza, R.; Rodríguez-Morales, J.A.; Escamilla-Montes, R.; Pool, H. Alginate microcapsules as delivery and protective systems of Bacillus licheniformis in a simulated shrimp’s digestive tract. Aquaculture 2021, 540, 736675.
- Ebrahimnejad, P.; Khavarpour, M.; Khalilid, S. Survival of Lactobacillus acidophilus as probiotic bacteria using chitosan nanoparticles. Int. J. Eng. 2017, 30, 456–463.
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M. Improving Survivability of Lactobacillus plantarum in Alginate-Chitosan Beads Reinforced by Na-Tripolyphosphate Dual Cross-Linking. LWT-Food Sci. Technol. 2018, 97, 440–447.
- Li, R.; Zhang, Y.; Polk, D.B.; Tomasula, P.M.; Yan, F.; Liu, L.S. Preserving Viability of Lactobacillus rhamnosus GG in Vitro and in Vivo by a New Encapsulation System. J. Control. Release 2016, 230, 79–87.
- Dafe, A.; Etemadi, H.; Dilmaghani, A.; Mahdavinia, G.R. Investigation of Pectin/Starch Hydrogel as a Carrier for Oral Delivery of Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 536–543.
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of Novel Carboxymethyl Cellulose/k-Carrageenan Blends as an Enteric Delivery Vehicle for Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307.
- Liao, N.; Luo, B.; Gao, J.; Li, X.; Zhao, Z.; Zhang, Y.; Ni, Y.; Tian, F. Oligosaccharides as Co-Encapsulating Agents: Effect on Oral Lactobacillus fermentum Survival in a Simulated Gastrointestinal Tract. Biotechnol. Lett. 2019, 41, 263–272.
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, N.F.; El-Sayed, H.S. Survivability of Alginate-Microencapsulated Lactobacillus plantarum during Storage, Simulated Food Processing and Gastrointestinal Conditions. Heliyon 2020, 6, e03541.
- Dehkordi, S.S.; Alemzadeh, I.; Vaziri, A.S.; Vossoughi, A. Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl. Biochem. Biotechnol. 2020, 190, 182–196.
- Rather, S.A.; Akhter, R.; Masoodi, F.A.; Gani, A.; Wani, S.M. Effect of Double Alginate Microencapsulation on in vitro Digestibility and Thermal Tolerance of Lactobacillus plantarum NCDC201 and L. Casei NCDC297. LWT-Food Sci. Technol. 2017, 83, 50–58.
- Safeer Abbas, M.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Ullah, Q.; Elahi, S.; Ateeq, H.; Shah, Y.A.; et al. Probiotic viability as affected by encapsulation materials: Recent updates and perspectives. Int. J. Food Prop. 2023, 26, 1324–1350.
- Yin, M.; Chen, M.; Yuan, Y.; Liu, F.; Zhong, F. Encapsulation of Lactobacillus rhamnosus GG in whey protein isolate-shortening oil and gum Arabic by complex coacervation: Enhanced the viability of probiotics during spray drying and storage. Food Hydrocoll. 2023, 146, 109252.
- De Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349.
- Ghadermazi, R.; Asl, A.K.; Tamjidi, F. Optimization of whey protein isolate-quince seed mucilage complex coacervation. Int. J. Biol. Macromol. 2019, 131, 368–377.
- Hernandez-Rodriguez, L.; Lobato-Calleros, C.; Pimentel-Gonzalez, D.J.; Vernon-Carter, E.J. Lactobacillus plantarum Protection by Entrapment in Whey Protein Isolate: κ-Carrageenan Complex Coacervates. Food Hydrocoll. 2014, 36, 181–188.
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-Encapsulation and Characterisation of Omega-3 Fatty Acids and Probiotic Bacteria in Whey Protein Isolate-Gum Arabic Complex Coacervates. J. Funct. Foods. 2015, 19, 882–892.
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth Adaptation of Probiotics in Biopolymer-Based Coacervate Structures to Enhance Cell Viability. LWT-Food Sci. Technol. 2017, 77, 282–289.
- Mao, L.; Pan, Q.; Yuan, F.; Gao, Y. Formation of Soy Protein Isolate-Carrageenan Complex Coacervates for Improved Viability of Bifidobacterium longum during Pasteurization and in vitro Digestion. Food Chem. 2019, 276, 307–314.
- da Silva, T.M.; de Deus, C.; Fonseca, B.d.S.; Lopes, E.J.; Cichoski, A.J.; Esmerino, E.A.; Silva, C.d.B.d.; Muller, E.I.; Flores, E.M.M.; de Menezes, C.R. The Effect of Enzymatic Crosslinking on the Viability of Probiotic Bacteria (Lactobacillus acidophilus) Encapsulated by Complex Coacervation. Food Res. Int. 2019, 125, 108577.
- Zhao, M.; Huang, X.; Zhang, H.; Zhang, Y.; Ganzle, M.; Yang, N.; Nishinari, K.; Fang, Y. Probiotic Encapsulation in Water-in-Water Emulsion via Heteroprotein Complex Coacervation of Type-A Gelatin/Sodium Caseinate. Food Hydrocoll. 2020, 105, 105790.
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic food compounds encapsulation by ionic gelation. Curr. Opin. Food Sci. 2017, 15, 50–55.
- Liu, Y.; Yang, G.; Zou, D.; Hui, Y.; Nigam, K.; Middelberg, A.P.; Zhao, C.X. Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery. Ind. Eng. Chem. Res. 2019, 59, 4134–4149.
- Modarres-Gheisari, S.M.M.; Gavagsaz-Ghoachani, R.; Malaki, M.; Safarpour, P.; Zandi, M. Ultrasonic nano-emulsification—A review. Ultrason. Sonochem. 2019, 52, 88–105.
- Walia, N.; Dasgupta, N.; Ranjan, S.; Ramalingam, C.; Gandhi, M. Methods for nanoemulsion and nanoencapsulation of food bioactives. Environ. Chem. Lett. 2019, 17, 1471–1483.
- Han, S.Y.; Nguyen, D.T.; Kim, B.J.; Kim, N.; Kang, E.K.; Park, J.H.; Choi, I.S. Cytoprotection of probiotic Lactobacillus acidophilus with artificial nanoshells of nature-derived eggshell mem-brane hydrolysates and coffee melanoidins in single-cell nanoencapsulation. Polymers 2023, 15, 1104.
- Ghorbani, S.; Maryam, A. Encapsulation of lactic acid bacteria and Bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model. J. Food Process. Preserv. 2021, 45, e16048.
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750.
- Li, S.; Fan, L.; Li, S.; Sun, X.; Di, Q.; Zhang, H.; Li, B.; Liu, X. Validation of layer-by-layer coating as a procedure to enhance Lactobacillus plantarum survival during in vitro digestion, storage, and fermentation. J. Agric. Food Chem. 2023, 71, 1701–1712.
- Horuz, T.İ.; Belibağlı, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93.
- Moncada, M.; Astete, C.; Sabliov, C.; Olson, D.; Boeneke, C.; Aryana, K.J. Nano spray-dried sodium chloride and its effects on the microbiological and sensory characteristics of surface-salted cheese crackers. J. Dairy Sci. 2015, 98, 5946–5954.
- Vinitha, K.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Size-dependent enhancement in salt perception: Spraying approaches to reduce sodium content in foods. Powder Technol. 2021, 378, 237–245.
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Farag, M.A.; Varzakas, T.; Lorenzo, J.M. Nanotechnology. In Sustainable Production Technology in Food; Lorenzo, J.M., Munecata, P.E.S., Barba, F.J., Eds.; Elsevier: Cambridge, MA, USA, 2021; pp. 179–202.
- Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers 2023, 15, 2402.
- Xue, J.; Wu, T.; Dai, Y.Q.; Xia, Y.N. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415.
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control. Release 2022, 349, 184–205.
- Xu, C.; Ma, J.; Liu, Z.; Wang, W.; Liu, X.; Qian, S.; Chen, L.; Gu, L.; Sun, C.; Hou, J.; et al. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023, 402, 134253.
- Ma, J.; Li, T.; Wang, Q.; Xu, C.; Yu, W.; Yu, H.; Wang, W.; Feng, Z.; Chen, L.; Hou, J.; et al. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chem. 2023, 413, 135680.
- Brandelli, A.; Reque, P.M. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10.
- Dimopoulos, G.; Katsimichas, A.; Oreopoulou, V.; Taoukis, P.; Tsimogiannis, D. Cell permeabilization processes for improved encapsulation of oregano essential oil in yeast cells. J. Food Eng. 2021, 294, 110408.
- Errenst, C.; Kilzer, A.; Petermann, M. Encapsulation of limonene in yeast cells using the concentrated power from technology. J. Supercrit. Fluids 2021, 168, 105076.
- Fumi, M.D.; Trioli, G.; Colombi, M.G.; Colagrande, O. Immobilization of Saccharomyces cerevisiae in calcium alginate gel and its application to bottle-fermented sparkling wine production. Am. J. Enol. Vitic. 1988, 39, 267–272.
- Colagrande, O.; Silva, A.; Fumi, M.D. Recent applications of biotechnology in wine production. Biotechnol. Prog. 1994, 10, 2–18.
- Moreno-García, J.; García-Martínez, T.; Mauricio, J.C.; Moreno, J. Yeast immobilization systems for alcoholic wine fermentations: Actual trends and future perspectives. Front. Microbiol. 2018, 9, 241.
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Mauricio, J.C. Use of a novel immobilization yeast system for winemaking. Biotechnol. Lett. 2005, 27, 1421–1424.
- Peinado, R.A.; Moreno, J.J.; Villalba, J.M.; González-Reyes, J.A.; Ortega, J.M.; Mauricio, J.C. Yeast biocapsules: A new immobilization method and their applications. Enzyme Microb. Technol. 2006, 40, 79–84.
- García-Martínez, T.; Puig-Pujol, A.; Peinado, R.A.; Moreno, J.; Mauricio, J.C. Potential use of wine yeasts immobilized on Penicillium chrysogenum for ethanol production. J. Chem. Technol. Biotechnol. 2012, 87, 51–359.
- García-Martínez, T.; Moreno, J.; Mauricio, J.C.; Peinado, R. Natural sweet wine production by repeated use of yeast cells immobilized on Penicillium chrysogenum. LWT-Food Sci. Technol. 2015, 61, 503–509.
- Puig-Pujol, A.; Bertran, E.; García-Martínez, T.; Capdevila, F.; Mínguez, S.; Mauricio, J.C. Application of a new organic yeast immobilization method for sparkling wine production. Am. J. Enol. Vitic. 2013, 64, 386–394.
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29.
- Ogawa, M.; Carmona-Jiménez, P.; García-Martínez, T.; Jorrín-Novo, J.V.; Moreno, J.; Rey, M.D.; Moreno-García, J. Use of yeast biocapsules as a fungal-based immobilized cell technology for Indian Pale Ale-type beer brewing. Appl. Microbiol. Biotechnol. 2022, 106, 7615–7625.
- Liu, M.; Qin, X.; Wu, X. Study on the technology of brewing red raspberry wine by using new immobilized yeast technology. Sci. Rep. 2022, 12, 21344.
- García-Martínez, T.; Peinado, R.A.; Moreno, J.; García-García, I.; Mauricio, J.C. Co-culture of Penicillium chrysogenum and Saccharomyces cerevisiae leading to the immobilization of yeast. J. Chem. Technol. Biotechnol. 2011, 86, 812–817.
- López-Menchero, J.R.; Ogawa, M.; Mauricio, J.C.; Moreno, J. Effect of calcium alginate coating on the cell retention and fermentation of a fungus-yeast immobilization system. LWT-Food Sci. Technol. 2021, 144, 111250.
- Luquez-Caravaca, L.; Ogawa, M.; Rai, R.; Nitin, N.; Moreno, J.; Garcia-Martinez, T.; Mauricio, J.C.; Jimenez-Uceda, J.C.; Moreno-Garcia, J. Yeast cell vacuum infusion into fungal pellets as a novel cell encapsulation methodology. Appl. Microbiol. Biotechnol. 2023, 107, 5715–5726.
- Esposto, B.S.; Jauregi, P.; Martelli-Tosi, M.; Tapia-Blacido, D.R. Liposomes vs. chitosomes: Encapsulating food bioactives. Trends Food Sci. Technol. 2021, 108, 40–48.
- Ghanbarzadeh, B.; Hamishehkar, H.; Mohammadi, M.; Nobari-Azar, F.A.; Pezeshki, A. Nanostructured lipid carriers: Promising delivery systems for encapsulation of food ingredients. J. Agric. Food Res. 2020, 2, 100084.
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll. 2020, 105, 105774.
- Adeel, M.; Afzaal, M.; Saeed, F.; Ahmed, A.; Mahmood, K.; Abbas shah, Y.; Ateeq, H.; Sibat, A.; Khan, M.R.; Busquets, R. Encapsulation of probiotic bacteria using polyelectrolytes stabilized nanoliposomes for improved viability under hostile conditions. J. Food Sci. 2023, 88, 3839–3848.
- Feng, B.; Tan, C.; Xia, S.; Xia, W.; Zhang, X. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784.
- Farias, T.G.S.; Ladislau, H.F.L.; Stamford, T.C.M.; Medeiros, J.A.C.; Soares, B.L.M.; Arnaud, T.M.S.; Stamford, T.L.M. Viabilities of Lactobacillus rhamnosus ASCC 290 and Lactobacillus casei ATCC 334 (in free form or encapsulated with calcium alginate-chitosan) in yellow mombin ice cream. LWT-Food Sci. Technol. 2019, 100, 391–396.
- Liu, Y.; Dong, L.; Li, Y.; Chen, Q.; Wang, L.; Farag, M.A.; Liu, L.; Zhan, S.; Wu, Z.; Liu, L. Soy protein isolate-citrus pectin composite hydrogels induced by TGase and ultrasonic treatment: Potential targeted delivery system for probiotics. Food Hydrocoll. 2023, 143, 108901.
- Zhang, F.; Wang, R.; Zhang, L.; Yan, L.; Jia, Y.; Yang, J.; Wang, X.; Lü, X. Enhanced viability of probiotics in composite hydrogel beads. J. Food Eng. 2023, 111621.
- Mariappan, B.; Prakash, S.; Binesh, A. Probiotic nanoparticles for food. In Recent Advances in Aquaculture Microbial Technology; Mathew, J., Radhakrishnan, E.K., Midhun, S.J., Kumar, A., Eds.; Academic Press: Boston, MA, USA, 2023; pp. 307–338.
More