2. Burden of Disease
The burden of disease refers to the impact of health problems on a given population, typically considering clinical, economic, and/or political indicators and often expressed in terms of the costs of disease to individuals, healthcare systems, and/or society
[3][8]. Estimating the global burden of RDs is a challenging task for at least three reasons: the coexistence of multiple definitions across the globe, the diversity of conditions that the concept encompasses, and the limited capacity to systemically track and diagnose these populations in several countries and regions. Nonetheless, understanding the burden of RDs is fundamental to informing public policy and defining priorities
[4][6].
2.1. The Epidemiological and Economic Impact at the Global Level
According to a report from the World Economic Forum published in 2020, it is estimated that between 350 and 475 million people are affected by RDs globally
[5][9], many of whom are children (approximately 50% of the people affected by RDs)
[6][10]. This has significant consequences both in terms of lives lost and the social and economic burden on families and caregivers. It is estimated that RDs are responsible for 35% of deaths in the first year of life
[7][11] and that 30% of children with an RD will not live to see their fifth birthday
[6][10]. Caring for RD patients is time-intensive, emotionally stressful, physically demanding, and economically straining
[8][9][10][12,13,14]. Whether due to the impact on patients’ and caregivers’ capacity to work or the high out-of-pocket expense of RD treatments, many families struggle to make ends meet
[11][12][13][14][15,16,17,18].
The economic impact of RDs is large and includes direct costs of treatment, along with non-clinical supporting costs and an overall cost of lost productivity for the patient and their caregivers. According to a meta-analysis of studies published between 2010 and 2017 (mostly from North American or European countries), direct health costs account for most of the economic impact of RDs, with great cost variability across RDs and countries
[15][19]. A systematic review of cost-of-illness studies for RDs found scarce evidence and high variability across conditions. For example, the total cost per patient per year for haemophilia was estimated to range from EUR 1101 to EUR 178,796, making it difficult to compare to the economic impact of other common conditions
[16][20].
The high economic impact of RDs is closely linked to the costs associated with drugs and care. Some of the most expensive drugs on the market are targeted to treat RDs
[17][21]. Orphan drugs cost roughly five times more than non-orphan drugs
[18][22], which poses challenges and concerns in terms of the sustainability of health systems
[19][23]. However, the high economic impact of RDs is also driven by the cost of care. Evidence indicates that costs are higher in a scenario without treatment when compared to a scenario with treatment
[20][24].
Considering the estimated prevalence of 100,000 people, the most common RDs globally are narcolepsy (50), primary biliary cholangitis (40), Fabry disease (30), cystic fibrosis (25), hemophilia (20), spinal muscular atrophy (13), and retinal dystrophy (13)
[21][25].
2.2. The Epidemiological and Economic Impact per Region
Many countries have only estimations for prevalence data available, as RDs are commonly under-diagnosed.
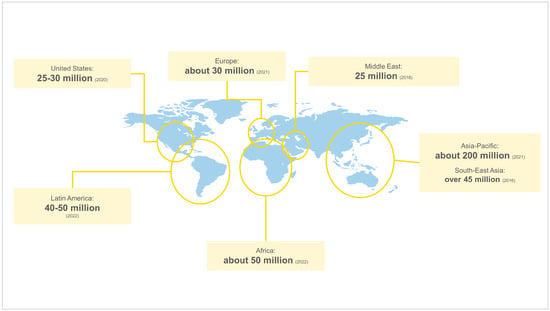
Figure 1 presents the estimated number of people affected by RDs across regions. Asia-Pacific is the region with the highest estimated absolute value of people affected by RDs, with around 200 million people living with these conditions
[22][26]. Anecdotal evidence suggests that this might be linked to the population size of the region (which is the largest), as well as practices present in some countries (such as consanguineal marriages and maternal and paternal advanced age). Africa has around 50 million people affected by RDs
[23][27], similar to Latin America, where an estimated 40 to 50 million people are living with an RD
[24][25][28,29]. Among countries in the region, the highest burdens are observed in Brazil, Mexico, and Argentina
[26][30]. RDs affect around 30 million people in Europe
[27][31], between 25 and 30 million people in the United States
[28][29][32,33], and 25 million people in the Middle East
[30][34].
Figure 1. Number of people affected by RDs across regions. Source: Elaborated by authors based on overviewed literature
[22][23][24][25][26][27][28][29][30][26,27,28,29,30,31,32,33,34]. The estimated values depend on data availability and local diagnostic and tracking capacity.
The prevalence of RDs varies across geographic areas due to population genetic diversity, as well as environmental and behavioral factors
[31][32][33][35,36,37]. However, it is important to note that the true prevalence of RDs depends on standardized, well-established, and specific diagnostic criteria, which vary across countries and regions
[34][38]. Low-resource settings may face limited clinical information, lack of reliable epidemiological data, inappropriate diagnostic knowledge and resources, and poor monitoring systems
[35][39], leading to an underestimation of RD prevalence. Compared to Europe or the United States, evidence indicates a lack of RD epidemiological information in many parts of the world, such as India, China, South America, and Africa
[4][6]. Acknowledging this reality is particularly important when comparing RD prevalence among and within regions. In the African continent, for example, it may be reasonable to speculate that a higher prevalence of RDs in South Africa, when compared to neighboring countries, could be associated with having a more developed tracking system, although
rwe
searchers found no robust evidence to support this claim.
In North America, data from the United States highlights the high economic burden of RDs and how it associates to direct as well as indirect and non-medical costs. RD expenditure in the USA was estimated to be USD 966 billion in 2019, from which USD 418 billion corresponded to direct medical costs and USD 548 billion to indirect and non-medical costs
[36][40]. To put this into perspective, these values are higher than those estimated for some chronic diseases, such as diabetes (USD 966 billion for 15 million RD patients vs. USD 327 billion for 24.7 million diabetes patients)
[36][40]. The leading categories for direct medical costs include inpatient and outpatient services, other ancillary services, and prescription drugs. For indirect and non-medical costs, the leading categories are absenteeism, presenteeism, forced retirement, and healthcare services not covered by insurance. Nonetheless, while direct healthcare costs are high, the cost of treating RD patients might be even higher. A systematic review of the costs of sickle cell disease (SCD) and treatments in the United States found that long-term treatment of SCD can decrease total medical costs, as complications lead to increased hospital visits and healthcare utilization
[37][41].
Furthermore, the financial burden incurred due to out-of-pocket RD treatment spending might put patients and their families in economic vulnerability. Considering that the median household income in the United States was USD 67,521 in 2020
[38][42] and that out-of-pocket RD treatment costs were estimated at approximately USD 26,887 per-person excess cost when compared to a person without an RD
[36][40], the medical costs an average RD patient faces represent approximately 39% of the total median household income.
In Latin America, evidence also demonstrates the economic burden of RDs due to direct and indirect costs on health systems. For example, in Mexico, the direct and indirect annual cost per patient with hemophilia, including diagnosis, follow-up, prophylaxis, treatment, and hospitalization, was estimated at USD 332,458 in 2019, out of which a substantial share depended on the use of hemostatic factors (the annual cost per patient with inhibitors was 4.2 times higher than that for patients without inhibitors)
[39][43]. Like North America, the high direct economic burden is often driven by the high costs of medicines. For example, in Ecuador, it was estimated that the treatment per patient with hemophilia had a monthly cost of approximately USD 13,172 in 2017
[40][44]. Aside from direct costs, patients and their families often shoulder significant indirect and intangible costs associated with RDs. This is, for example, the case of patients with mucopolysaccharidosis VI in Colombia, who face the responsibility of costs associated with complications, frequent healthcare encounters, and caregiver dedication
[12][16]. Patients with RDs, although not very numerous, also represent a high economic burden on countries’ national budgets. For example, in Peru, the Ministry of Health spent USD 33 million in care for over 42,000 people living with a rare or orphan disease in 2019
[41][45], and in Colombia, the economic burden of atypical hemolytic uremic syndrome treatment was USD 3,907,891 for only 18 patients in 2019
[42][46].
In Europe, one study published in 2016 analyzed the social and economic costs of RDs and estimated that drugs represented nearly 90% of direct healthcare costs (costs attributable to patient care, medical management of the disease, drugs, admissions, and complementary tests) in most of the analyzed countries
[43][47]. In fact, the share that orphan drugs represent in relation to total pharmaceutical expenditure has increased since 2000 across European countries. Orphan drugs expenditure reached approximately EUR 10.5 billion in 2017, while the total value spent on medicines that year was approximately EUR 147 billion, which represented 7.2% of total pharmaceutical expenditure (this share was around 4% in 2012 and less than 1% in 2005)
[44][48]. While orphan drug designation and marketing authorization are centralized in the European Union (EU), decisions on pricing, reimbursement, and funding for orphan medicines remain the responsibilities of Member States. This leads to uneven access to orphan medicines across Europe and a great variation in the costs of orphan drugs per patient
[45][49]. The differences in costs per RD patient across different European countries demonstrate that market access strategies are fragmented, impacting orphan and non-orphan medicines
[46][50]. This, in turn, affects the quality of care and treatment that RD patients receive
[47][48][51,52].
In Asia-Pacific countries, the affordability of drugs to treat RDs is one of the major issues faced, as the strain put on households by out-of-pocket RD treatment costs places patients and their families in a particularly vulnerable position, with many families being unable to afford treatment. In Australia, a study found that 45% of parents of children who have an RD are not able to cope with the costs associated with their children’s conditions, and 29% had to increase their working hours or take a second job
[13][17]. In China, one study found that over 90% of RD patients could not afford their costs of living in 2016 and that the medical expenditure of an individual with an RD was, on average, three times higher than his or her individual income
[14][18]. Moreover, in some countries, the lack of local production of RDs medicines and heavy reliance on imported drugs lead to higher costs of treatment, posing a higher financial burden on RD patients and health systems. This is, for example, the case in India
[49][50][53,54]. Finally, like other regions, evidence from some countries also indicates that drug expenditures account for most of the direct RD healthcare costs. For instance, in Taiwan, a 2019 study not only revealed that drug expenditures for the treatment of RDs increased from USD 13.24 million in 2003 to USD 121.98 million in 2014 (accounting for 2.31% of drug expenditures for the total population), but also that expenditure accounted for 70% and 89% of the total health expenditures for patients with RDs, respectively
[51][55].
In Africa and the Middle East, evidence is indicative of the need to have policies for RDs that are comprehensive and multidimensional. In this region, RDs are low on the health policy agenda, as demonstrated by the presence of underdiagnosis, lack of care, and lack of evidence, which result in substantially higher costs down the line
[52][56]. Moreover, in many countries, competing priorities, such as nutrition and communicable disease prevention, constrain the possibilities of health systems to provide adequate care for RD patients. For example, in South Africa, even though it is estimated that one in 15 people are affected by an RD
[53][57], directing the additional funds necessary to adequately manage RDs is limited by the lack of public investment in health
[53][57]. Like other regions, in the Middle East, patients with RDs face high costs to access medicines. For instance, in Saudi Arabia, the reimbursement system severely limits the coverage for orphan drugs, which, together with bureaucracy on imported goods, delays treatment and leads to disease deterioration
[54][58].
2.3. Gaps, Barriers, and Challenges
RDs are tremendously heterogeneous in their symptoms, progression, how they affect patients, and potential treatments. Due to the disease heterogeneity and geographic dispersion, there is a lack of reliable and significant evidence on their impact globally. Moreover, evidence of the economic impact of these diseases is still missing in many parts of the world. Most countries in the Asia-Pacific, Africa and the Middle East, and Latin America lack or have limited evidence of the economic impact of RDs.
The high costs of medicines represent a substantial share of the economic burden of RDs. There are methods and tools that can be employed to control costs, such as repurposing drugs for new indications. This is a timesaving, cost-efficient method that can accelerate the development of RDs treatments
[55][59]. A successful case of drug repurposing is the use of Gentamicin (an aminoglycoside antibiotic used in the treatment of several gram-negative infections) to treat Duchenne muscular dystrophy
[56][60]. Clinical studies have demonstrated its effectiveness and long-term safety in treating this RD
[57][61]. Despite its potential, this approach can be hampered by different challenges such as financial and intellectual property considerations, the regulatory path, and challenges in performing clinical trials
[56][60].
In a general sense, there is a paucity of cost-of-illness studies on RDs
[58][62]. The limited availability of medical history and epidemiologic data regarding RDs, as well as a standardized methodological approach to calculate cost-of-illness, constrains the possibility to estimate direct and indirect costs associated with RDs and, consequently, the estimation of potential economic benefits of treatment
[59][60][63,64]. Moreover, cost-of-illness studies of RDs rarely analyze the outcomes or benefits of possible treatments
[61][65]. While this is true, evidence also indicates that economic benefits derived from RD therapies will likely manifest in the long term, such as reducing both direct and indirect expenditures
[60][64]. One study on direct, indirect, and mortality-related costs for a sample of 24 RDs in the United States found that total costs per RD patient per year were 21.2% higher in a scenario without treatment, when compared to a scenario with treatment, highlighting the substantial value that access to RD treatment generates
[20][24].
There is also a lack of reliable, consistent, and multidimensional socioeconomic measures on the burden of disease to capture the full scope of the impact of RDs on patients, their families, their caregivers, and society at large. The absence of a common measure further limits the possibility of assessing the value of RD innovation using a multi-criteria approach (beyond cost-effectiveness). Decision-making regarding the assessment, financing, and reimbursement of RD innovation requires robust evidence-based analyses
[3][8]. Likewise, policy and budget planning to treat, diagnose, and care for RD patients also requires real-world data. The lack of interoperable RD surveillance systems and registries in many regions of the world (including the Asia-Pacific, Africa and the Middle East, and Latin America regions) particularly limits the capacity of decision makers to respond to the needs of the RD community. Overall, it is crucial to collect and communicate evidence on the burden of RDs that is compelling to regulators, policymakers, and payers, so they are equipped to understand the scale of the issue, acknowledge the needs of the population, and make decisions accordingly
[62][66].
Regarding funding, many countries continue to struggle with putting RDs on the policy agenda. This is especially true for lower-income countries with a high prevalence of communicable diseases. As for high-income countries, the main issue rests on the lack of organization of different funding programs, as well as a lack of dedicated RDs research funds
[63][67]. With few incentives and little support available for RDs research, decision makers struggle to measure and understand the return on investing in RDs.
Finally, the increasing number of novel RDs being identified requires a more holistic focus by patient organizations, to ensure patients with novel RDs count with the community support needed. According to evidence, approximately 50% of RDs do not have a disease-specific foundation/research group or community readily available
[64][68]. Patient organizations play a pivotal role in advocating for the development of new therapies. There is a need to continue empowering patient organizations to participate knowledgeably during reimbursement decision-making.