Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Mona Zou and Version 1 by Hans Van der Eecken.
A possible link between diet and cancer has long been considered, with growing interest in phytochemicals. Soy isoflavones have been associated with a reduced risk of prostate cancer in Asian populations. Of the soy isoflavones, genistein and daidzein, in particular, have been studied, but recently, equol as a derivative has gained interest because it is more biologically potent. Different mechanisms of action have already been studied for the different isoflavones in multiple conditions, such as breast, gastrointestinal, and urogenital cancers. Many of these mechanisms of action could also be demonstrated in the prostate, both in vitro and in vivo.
- isoflavone
- genistein
- daidzein
- equol
- prostate cancer
1. Soy—One Word, Different Worlds
Soybeans are the richest source of isoflavones, and when soybeans are fermented, isoflavone aglucon is produced through the removal of a glucoside group [10][1]. The most well-known aglucones are genistein, daidzein, and glycitein. Glycitein differs slightly from genistein and daidzein due to a separate O-methyl group and represents only 5–10% of total isoflavones (Figure 1).
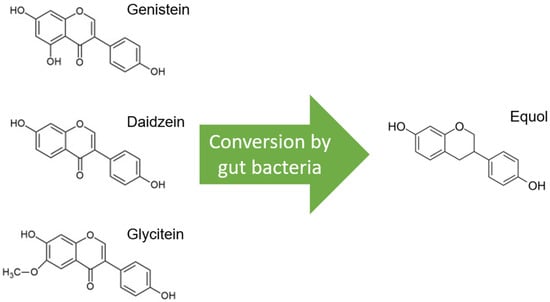
Figure 1.
Molecular structures of the isoflavones genistein, daidzein, and glycitein, which are very similar, but note the distinct O-methyl group in glycitein. Only daidzein is converted to equol by gut bacteria.
Glycitein has, therefore, been less studied and is therefore excluded from this review, although its beneficial effects have already been described, especially in gastrointestinal cancers and breast cancer [11][2]. Only daidzein, and not genistein or glycitein, is converted to equol by the microflora in the gut, and this is estimated in only 20–35% of the Western population versus 60% of the Asian population (Figure 1) [12][3]. Only S-equol, an enantiomer with selective affinity for estrogen receptor β (ER-β), is made by these gut bacteria, in contrast to R-equol, which has more affinity for estrogen receptor α (ER-α) [13,14][4][5]. Equol differs from genistein and daidzein in chemical characteristics, and consequently, equol has some other features, such as greater antioxidant activity [15][6].
The biological effects of genistein, daidzein, and equol are expressed via interaction with multiple and complex cellular pathways. There is interaction with androgen- and estrogen-driven pathways, cell proliferation and cell cycle, angiogenesis, and metastasis. In addition, these molecules have anti-inflammatory and antioxidant properties and possess potential anticancer epigenetic activity. The “characteristics of cancer” were used as a conceptual guide in this review [16][7].
It is important to stress that most of the results in this review were obtained with in vitro studies, where it is generally believed that plant molecules such as isoflavones may have a more pronounced effect when applied directly to cell culture versus in vivo. Therefore, there is also a difference in dosage, and smaller doses are used on average in vitro, ranging from low concentrations (0.1–5 µM) to medium (10–50 µM) and higher (200 µM) concentrations. There may also be a difference depending on the cell lines used. In contrast, animal models use different dose levels, ranging from low (5 mg/kgBW to 20 mg/kgBW) to higher doses (100 mg/kgBW to 250 mg/kgBW) or even more, spread over one or more intakes per day (BW = body weight). The above comment applies to the different sections highlighted below, and therefore, it is always noted whether the results were reported in cell lines or in vivo.
2. The Modification of Androgen- and/or Estrogen-Mediated Carcinogenesis
Of the two known estrogen receptors in humans (ER-α and ER-β), ER-β is predominantly found in the prostate [83][8]. The structure of genistein and equol is very similar to that of estrogen, so both compete with estrogens to bind to the ER and modulate ER function (Figure 2) [14,84][5][9].
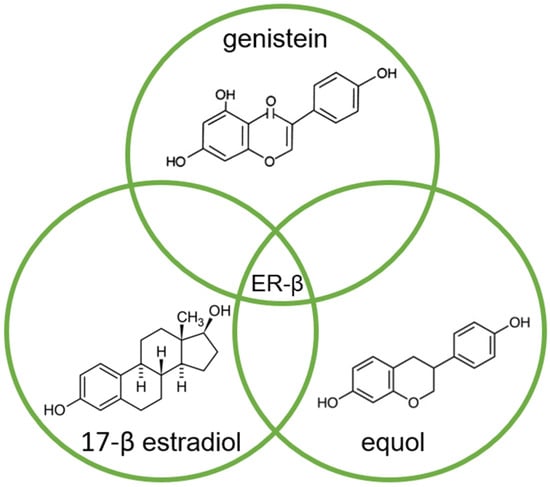
Figure 2.
Molecular structures of genistein, equol, and 17-β estradiol, which share a common feature of high affinity for binding to the estrogen receptor (ER-β), due to their high similarity.
The affinity of genistein and equol for binding to ER-β is similar to the affinity of 17-β estradiol (E2) for this receptor, although the concentration of genistein required to induce transcription is 104 times higher compared to E2 [84][9].
There also is a clear link between the ER-β and the androgen receptor (AR): activation of the ER-β downregulates the AR, which results in a reduced response of prostate tissue to androgen stimulation [17,85,86][10][11][12]. In turn, this effect causes reduced production of the prostate-specific antigen (PSA) [87][13]. In the case of androgen-sensitive prostate cancer cells, low concentrations (0.1–5 µM) of genistein are sufficient to lower the PSA level, whereas in androgen-independent cell lines, concentrations should be markedly higher (10–50 µM) [38,88][14][15]. This isoflavone-induced downregulation of the AR was also demonstrated in animal models (diet containing 100–500 mg/kgBW genistein daily), which in turn reduced tumor growth [17,89,90,91][10][16][17][18].
Equol has a unique ability to bind dihydrotestosterone (DHT), thus sequestering DHT and preventing binding to the AR, which has anti-androgenic effects [92][19]. Via a ubiquitin ligase (Skp2) and a proteasomal pathway, equol also causes AR degradation (=ubiquitination) [24][20]. In turn, genistein also causes AR degradation via ubiquitination, but with the help of heat shock proteins (HSP), including HSP90 [18,19][21][22].
In addition to all these described mechanisms of action via AR interaction at the cellular level, there may also be an effect on AR gene expression. In particular, genistein in vitro (10 µM) is thought to be effective in this way, with a decrease in AR mRNA [5][23]. However, in a study with male rats, equol at a dose of 100 to 250 mg/kgBW/day was found to be unable to change AR mRNA expression in the prostate [20][24].
3. Inhibition of Cancer Cell Growth
In human prostate cancer cells, genistein was able to inhibit cell growth independent of AR status, and this was seen in both the androgen-independent cell lines PC-3 and DU-145 and in the androgen-sensitive LNCaP line [40,45,47,48,91,93,94,95,96][18][25][26][27][28][29][30][31][32]. An important mechanism induced by high doses of genistein (>10 µM) is the inhibition of growth factor tyrosine kinase (TK) activity [25][33]. Inhibitors of these TKs nowadays play a prominent role in the treatment of several malignancies [97][34], and TKs might be interesting targets for PCa-targeted therapies [6][35]. Concerning PCa, receptor-mediated TK activation is considered to be one of the mechanisms for acquiring the androgen-independent (or castration-refractory) status [98,99][36][37]. However, in in vitro experiments, the dose of genistein needed to achieve this effect reached the upper limit of physiologically attainable doses (>10 µM) [25][33], a situation that is not realistic in vivo [10][1].
PCa cells often have an increased expression of the ErbB receptor family (proteins), such as the epidermal growth factor receptor (EGFR or ErbB-1), ErbB-2 (also called HER2), and ErbB-3 (also named HER3) [25][33]. Genistein at a higher dose (100–200 µM) is a potent inhibitor of the EGFR in the androgen-independent DU-145 cell line [26][38]. In animal models, the inhibition of the expression of both EGFR and ErbB-2 receptors by genistein (0.05–1 mg/g diet) was demonstrated [27][39].
Insulin growth factor 1 (IGF-1) is also thought to play an important role mainly via promoting progression and metastasis but also blocking apoptosis [100,101,102][40][41][42]. TKs also play a role in this, as they are activated when IGF-1 binds to its membrane receptor. As a result, the insulin receptor substrate (IRS-1) is phosphorylated [32][43]. In turn, PI3K/AKT and RAS/MAPK are activated, resulting in cell proliferation. This IGF-1-stimulated cell growth was inhibited by genistein in PC-3, LNCaP, and DU-145 cell lines at rather average doses (25–40 µM) [28,29,30][44][45][46]. Moreover, genistein inhibits the phosphorylation of other mediators such as glycogen synthase kinase-3β (GSK-3β), Src, FOXO3a, Akt, and p70S6k, leading to the downregulation of AR [21,22,23][47][48][49].
FOXO (forkhead box O) proteins can suppress tumors, but these may themselves be inhibited through mitogen-activated protein kinase (MAPK)-mediated phosphorylation [103,104][50][51]. This phosphorylation is inhibited by genistein, equol, and daidzein, which can increase FOXO proteins [29,31][45][52].
Another pathway involved in the progression of PCa is the Wnt/β-catenin pathway. Upon the presence of the Wnt ligand, cytoplasmic β-catenin is phosphorylated and freed from its complex. At the level of the cell nucleus, binding to the transcription factor T-cell factor-4 (TCF-4) occurs, resulting in the activation of transcription of genes responsible for cell proliferation (c-Myc and cyclin D1) [33][53]. Blocking this pathway in PC-3 cells with genistein (100 µM) resulted in the marked suppression of PCa cell growth [34,35][54][55].
Poly(ADP-ribose)polymerase (PARP)-inhibitors are a new kid on the block in the treatment of metastatic PCa by acting on apoptotic cell death. In PC-3 and LNCaP cells, genistein induces cleavage of PARP [48][28]. Cleavage occurs by caspase 3, on which genistein acts specifically, as demonstrated in PC-3 cell lines [36][56]. In these PC-3 cells, genistein, even at doses of 50 µM, decreased the activity of Akt kinase and reduced phosphorylation of the Akt protein when compared to PC-3 cells that were not treated with genistein [38][14]. This reduced Akt phosphorylation leads to the decreased antiapoptotic function of the protein. This gives rise to the hypothesis that genistein acts as an initiator of apoptotic cell death, at least in PC-3 cells. Additionally, PC-3 cells treated with genistein showed a reduction in mRNA levels of survivin and protease-activated receptor 2 (PAR-2) that delay apoptosis. In contrast, mRNA levels for elafin were increased, which increases apoptosis [37][57].
Two key proteins involved in maintaining balance in cellular life are Bcl-2 and Bax. Bcl-2 inhibits cellular apoptosis, while Bax stimulates it via stimulation of the mitochondria with the release of cytochrome C and activation of caspases. Genistein (25 µM) stimulates Bax and suppresses Bcl-2, giving a stronger ratio for Bax and inducing apoptosis [38][14].
Genistein can also induce apoptosis through interfering with the proteasome. This is a protein complex that degrades proteins that are no longer needed or damaged via proteolysis. Thus, proteins that promote cell cycle regulation can be degraded, leading to apoptosis [39][58]. A simultaneous accumulation of ubiquitinated proteins was seen, including the cyclin-dependent kinase (CDK) inhibitor p27, the inhibitor of nuclear factor-Kβ (NF-Kβ), and the Bax protein.
Nuclear factor-Kβ (NF-Kβ) are transcription factors that, when activated, can protect against apoptosis. They do this via binding to the so-called Kβ sites of DNA (5′-GGGRNYY YCC-3′), and this process is mediated by the IKB protein [42,43,105][59][60][61]. Genistein appears to inhibit the binding of NK-Kβ to DNA, which could be demonstrated in the different prostate cancer cell lines at a moderate dose of 50 µM [37,40,41][25][57][62]. This inhibition is based on the inhibition of the phosphorylation of IKB. Consequently, the effect of NK-Kβ on DNA is prevented, and protection from apoptosis is countered [41][62]. Moreover, there is a link between NF-KB and the Akt pathway. Akt enhances the degradation of IKB and thereby induces NF-Kβ activity [106][63]. As mentioned above, genistein has been demonstrated to inhibit the Akt signaling pathway and NF-Kβ activation through this mechanism [40][25].
Autophagy is viewed as a variant of programmed cell death in which cellular components are degraded by lysosomes [107][64]. In autophagy, the mammalian target of rapamycin (mTOR) has a signaling function, and it inhibits autophagy [108][65]. Soy isoflavones are able to suppress this mTOR signaling, and thus, autophagy is not inhibited, which was demonstrated in LNCaP and 22Rv1 PCa cells [44][66].
Furthermore, genistein (50 µM) also appears capable of downregulating telomerase reverse transcriptase, c-Myc RNA, and MDM2 oncogene, as was seen in the PCa cells DU-145 and LNCaP [45,46][26][67].
4. Effects on Cell Cycle Regulation
In PCa cells in culture, genistein inhibited growth with the arrest of the G2/M cell cycle, which was related to dose (5–50 µM). Simultaneously, there was downregulation of cyclin B1, upregulation of the growth-inhibitory protein p21WAF1, and induction of apoptosis [48][28]. In the androgen-independent cell line DU-145, there was also genistein-induced inhibition of the adaptor protein Shc, resulting in the inhibition of extracellular regulated kinase (ERK)1/2 activation. This inhibition was dose-dependent (100–200 µM) and without alteration in protein levels [26][38]. The inhibition of cell growth was also found in androgen-independent PC-3 cells, as well as in the androgen-sensitive LNCaP cells [48][28].
Cyclin-dependent kinases (CDKs) and cyclins are regulatory switches that allow the cell to move through the different phases of the cell cycle (from G1 to S-phase and from G2 to M-phase). Genistein interferes with these control switches and causes cell cycle arrest at various concentrations (20–200 µM) [48,49,109,110][28][68][69][70]. In the LNCaP cell line, genistein induced the G1 cell cycle arrest through the upregulation of CDK inhibitors [47][27]. In PCa cells, genistein induced the G2/M cell cycle arrest, combined with an increase in p21 and p27 and a decrease in cyclin B1 and CDK4 [30,48,49][28][46][68]. A similar mechanism at similar concentrations was seen for equol in PC-3 cells, along with an induction of apoptosis through the upregulation of Fas ligand (Fas) and expression of proapoptotic Bim [31][52]. The decreased expression of cyclin B1 was seen along with an increase in P53 proteins in both LNCaP and PC-3 cells with genistein and daidzein, even at low doses (5–10 µM) [111][71].
References
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and Their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98.
- Xiang, T.; Jin, W. Mechanism of Glycitein in the Treatment of Colon Cancer Based on Network Pharmacology and Molecular Docking. Lifestyle Genom. 2023, 16, 1–10.
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol—A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584.
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a Potent Ligand for Estrogen Receptor β, Is the Exclusive Enantiomeric Form of the Soy Isoflavone Metabolite Produced by Human Intestinal Bacterial Flora1–4. Am. J. Clin. Nutr. 2005, 81, 1072–1079.
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29.
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.G. Antioxidant Efficacy of Phytoestrogens in Chemical and Biological Model Systems. Arch. Biochem. Biophys. 1998, 360, 142–148.
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Bonkhoff, H. Estrogen Receptor Signaling in Prostate Cancer: Implications for Carcinogenesis and Tumor Progression. Prostate 2018, 78, 2–10.
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263.
- Fritz, W.A.; Wang, J.; Eltoum, I.-E.; Lamartiniere, C.A. Dietary Genistein Down-Regulates Androgen and Estrogen Receptor Expression in the Rat Prostate. Mol. Cell Endocrinol. 2002, 186, 89–99.
- Bektic, J.; Berger, A.P.; Pfeil, K.; Dobler, G.; Bartsch, G.; Klocker, H. Androgen Receptor Regulation by Physiological Concentrations of the Isoflavonoid Genistein in Androgen-Dependent LNCaP Cells Is Mediated by Estrogen Receptor β. Eur. Urol. 2004, 45, 245–251.
- Takahashi, Y.; Lavigne, J.A.; Hursting, S.D.; Chandramouli, G.V.R.; Perkins, S.N.; Barrett, J.C.; Wang, T.T.Y. Using DNA Microarray Analyses to Elucidate the Effects of Genistein in Androgen-Responsive Prostate Cancer Cells: Identification of Novel Targets. Mol. Carcinog. 2004, 41, 108–119.
- Montgomery, B.T.; Young, C.Y.-F.; Bilhartz, D.L.; Andrews, P.E.; Thompson, N.F.; Tindall, D.J.; Prescott, J.L. Hormonal Regulation of Prostate-Specific Antigen (PSA) Glycoprotein in the Human Prostatic Adenocarcinoma Cell Line, LNCaP. Prostate 1992, 21, 63–73.
- Sarkar, F.H.; Li, Y. Mechanisms of Cancer Chemoprevention by Soy Isoflavone Genistein. Cancer Metastasis Rev. 2002, 21, 265–280.
- Davis, J.N.; Muqim, N.; Bhuiyan, M.; Kucuk, O.; Pienta, K.J.; Sarkar, F.H. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int. J. Oncol. 2000, 16, 1091–1098.
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.E.; Greenberg, N.M.; Elgavish, A. Genistein in the Diet Reduces the Incidence of Poorly Differentiated Prostatic Adenocarcinoma in Transgenic Mice (TRAMP). Cancer Res. 2001, 61, 6777–6782.
- Pollard, M.; Wolter, W. Prevention of Spontaneous Prostate-Related Cancer in Lobund-Wistar Rats by a Soy Protein Isolate/Isoflavone Diet. Prostate 2000, 45, 101–105.
- Peterson, G.; Barnes, S. Genistein and Biochanin A Inhibit the Growth of Human Prostate Cancer Cells but Not Epidermal Growth Factor Receptor Tyrosine Autophosphorylation. Prostate 1993, 22, 335–345.
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.R.; Lephart, E.D.; Handa, R.J. Equol Is a Novel Anti-Androgen That Inhibits Prostate Growth and Hormone Feedback. Biol. Reprod. 2004, 70, 1188–1195.
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M.; et al. Equol Inhibits Prostate Cancer Growth through Degradation of Androgen Receptor by S-Phase Kinase-Associated Protein 2. Cancer Sci. 2016, 107, 1022–1028.
- Basak, S.; Pookot, D.; Noonan, E.J.; Dahiya, R. Genistein Down-Regulates Androgen Receptor by Modulating HDAC6-Hsp90 Chaperone Function. Mol. Cancer Ther. 2008, 7, 3195–3202.
- Sivoňova, M.; Kaplan, P.; Tatarkova, Z.; Lichardusova, L.; Dušenka, R.; Jurečekova, J. Androgen Receptor and Soy Isoflavones in Prostate Cancer (Review). Mol. Clin. Oncol. 2018, 10, 191–204.
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy Isoflavones and Prostate Cancer: A Review of Molecular Mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132.
- Loutchanwoot, P.; Srivilai, P.; Jarry, H. Lack of Anti-Androgenic Effects of Equol on Reproductive Neuroendocrine Function in the Adult Male Rat. Horm. Behav. 2014, 65, 22–31.
- Li, Y.; Sarkar, F.H. Inhibition of Nuclear Factor KappaB Activation in PC3 Cells by Genistein Is Mediated via Akt Signaling Pathway. Clin. Cancer Res. 2002, 8, 2369–2377.
- OUCHI, H.; ISHIGURO, H.; IKEDA, N.; HORI, M.; KUBOTA, Y.; UEMURA, H. Genistein Induces Cell Growth Inhibition in Prostate Cancer through the Suppression of Telomerase Activity. Int. J. Urol. 2005, 12, 73–80.
- Shen, J.-C.; Klein, R.D.; Wei, Q.; Guan, Y.; Contois, J.H.; Wang, T.T.Y.; Chang, S.; Hursting, S.D. Low-Dose Genistein Induces Cyclin-Dependent Kinase Inhibitors and G1 Cell-Cycle Arrest in Human Prostate Cancer Cells. Mol. Carcinog. 2000, 29, 92–102.
- Davis, J.N.; Singh, B.; Bhuiyan, M.; Sarkar, F.H. Genistein-induced Upregulation of P21WAF1, Downregulation of Cyclin B, and Induction of Apoptosis in Prostate Cancer Cells. Nutr. Cancer 1998, 32, 123–131.
- Geller, J.; Sionit, L.; Partido, C.; Li, L.; Tan, X.; Youngkin, T.; Nachtsheim, D.; Hoffman, R.M. Genistein Inhibits the Growth of Human-Patient BPH and Prostate Cancer in Histoculture. Prostate 1998, 34, 75–79.
- Hempstock; Kavanagh; George. Growth Inhibition of Prostate Cell Lines in Vitro by Phyto-Oestrogens. BJU Int. 1998, 82, 560–563.
- Shenouda, N.S.; Zhou, C.; Browning, J.D.; Ansell, P.J.; Sakla, M.S.; Lubahn, D.B.; MacDonald, R.S. Phytoestrogens in Common Herbs Regulate Prostate Cancer Cell Growth in Vitro. Nutr. Cancer 2004, 49, 200–208.
- Onozawa, M.; Fukuda, K.; Ohtani, M.; Akaza, H.; Sugimura, T.; Wakabayashi, K. Effects of Soybean Isoflavones on Cell Growth and Apoptosis of the Human Prostatic Cancer Cell Line LNCaP. Jpn. J. Clin. Oncol. 1998, 28, 360–363.
- Mahmoud, A.M.; Zhu, T.; Parray, A.; Siddique, H.R.; Yang, W.; Saleem, M.; Bosland, M.C. Differential Effects of Genistein on Prostate Cancer Cells Depend on Mutational Status of the Androgen Receptor. PLoS ONE 2013, 8, e78479.
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731.
- Bektic, J.; Guggenberger, R.; Eder, I.E.; Pelzer, A.E.; Berger, A.P.; Bartsch, G.; Klocker, H. Molecular Effects of the Isoflavonoid Genistein in Prostate Cancer. Clin. Prostate Cancer 2005, 4, 124–129.
- Graff, J.R.; Konicek, B.W.; McNulty, A.M.; Wang, Z.; Houck, K.; Allen, S.; Paul, J.D.; Hbaiu, A.; Goode, R.G.; Sandusky, G.E.; et al. Increased AKT Activity Contributes to Prostate Cancer Progression by Dramatically Accelerating Prostate Tumor Growth and Diminishing P27Kip1 Expression. J. Biol. Chem. 2000, 275, 24500–24505.
- Uzgare, A.R.; Isaacs, J.T. Enhanced Redundancy in Akt and Mitogen-Activated Protein Kinase-Induced Survival of Malignant versus Normal Prostate Epithelial Cells. Cancer Res. 2004, 64, 6190–6199.
- Bhatia, N.; Agarwal, R. Detrimental Effect of Cancer Preventive Phytochemicals Silymarin, Genistein and Epigallocatechin 3-Gallate on Epigenetic Events in Human Prostate Carcinoma DU145 Cells. Prostate 2001, 46, 98–107.
- Dalu, A.; Haskell, J.F.; Coward, L.; Lamartiniere, C.A. Genistein, a Component of Soy, Inhibits the Expression of the EGF and ErbB2/Neu Receptors in the Rat Dorsolateral Prostate. Prostate 1998, 37, 36–43.
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like Growth Factor (IGF)-I, IGF Binding Protein-3, and Cancer Risk: Systematic Review and Meta-Regression Analysis. Lancet 2004, 363, 1346–1353.
- Ozkan, E.E. Plasma and Tissue Insulin-like Growth Factor-I Receptor (IGF-IR) as a Prognostic Marker for Prostate Cancer and Anti-IGF-IR Agents as Novel Therapeutic Strategy for Refractory Cases: A Review. Mol. Cell Endocrinol. 2011, 344, 1–24.
- Gennigens, C.; Menetrier-Caux, C.; Droz, J.P. Insulin-Like Growth Factor (IGF) Family and Prostate Cancer. Crit. Rev. Oncol. Hematol. 2006, 58, 124–145.
- Wang, S.; DeGroff, V.L.; Clinton, S.K. Tomato and Soy Polyphenols Reduce Insulin-Like Growth Factor-I–Stimulated Rat Prostate Cancer Cell Proliferation and Apoptotic Resistance In Vitro via Inhibition of Intracellular Signaling Pathways Involving Tyrosine Kinase. J. Nutr. 2003, 133, 2367–2376.
- Werner, H.; Le Roith*, D. New Concepts in Regulation and Function of the Insulin-like Growth Factors: Implications for Understanding Normal Growth and Neoplasia. Cell. Mol. Life Sci. 2000, 57, 932–942.
- Takahashi, Y.; Lavigne, J.A.; Hursting, S.D.; Chandramouli, G.V.R.; Perkins, S.N.; Kim, Y.S.; Wang, T.T.Y. Molecular Signatures of Soy-Derived Phytochemicals in Androgen-Responsive Prostate Cancer Cells: A Comparison Study Using DNA Microarray. Mol. Carcinog. 2006, 45, 943–956.
- Rabiau, N.; Kossaï, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.-J.; Bernard-Gallon, D.J. Genistein and Daidzein Act on a Panel of Genes Implicated in Cell Cycle and Angiogenesis by Polymerase Chain Reaction Arrays in Human Prostate Cancer Cell Lines. Cancer Epidemiol. 2010, 34, 200–206.
- Oh, H.Y.; Leem, J.; Yoon, S.J.; Yoon, S.; Hong, S.J. Lipid Raft Cholesterol and Genistein Inhibit the Cell Viability of Prostate Cancer Cells via the Partial Contribution of EGFR-Akt/P70S6k Pathway and down-Regulation of Androgen Receptor. Biochem. Biophys. Res. Commun. 2010, 393, 319–324.
- Li, Y.; Wang, Z.; Kong, D.; Li, R.; Sarkar, S.H.; Sarkar, F.H. Regulation of Akt/FOXO3a/GSK-3beta/AR Signaling Network by Isoflavone in Prostate Cancer Cells. J. Biol. Chem. 2008, 283, 27707–27716.
- El Touny, L.H.; Banerjee, P.P. Identification of a Biphasic Role for Genistein in the Regulation of Prostate Cancer Growth and Metastasis. Cancer Res. 2009, 69, 3695–3703.
- Burgering, B.M.T. A Brief Introduction to FOXOlogy. Oncogene 2008, 27, 2258–2262.
- Roy, S.K.; Srivastava, R.K.; Shankar, S. Inhibition of PI3K/AKT and MAPK/ERK Pathways Causes Activation of FOXO Transcription Factor, Leading to Cell Cycle Arrest and Apoptosis in Pancreatic Cancer. J. Mol. Signal 2010, 5, 10.
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J.; et al. S-Equol, a Secondary Metabolite of Natural Anticancer Isoflavone Daidzein, Inhibits Prostate Cancer Growth In Vitro and In Vivo, Though Activating the Akt/FOXO3a Pathway. Curr. Cancer Drug Targets 2016, 16, 455–465.
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26.
- Liss, M.A.; Schlicht, M.; Kahler, A.; Fitzgerald, R.; Thomassi, T.; Degueme, A.; Hessner, M.; Datta, M.W. Characterization of Soy-Based Changes in Wnt-Frizzled Signaling in Prostate Cancer. Cancer Genom. Proteom. 2010, 7, 245–252.
- Lee, J.; Ju, J.; Park, S.; Hong, S.J.; Yoon, S. Inhibition of IGF-1 Signaling by Genistein: Modulation of E-Cadherin Expression and Downregulation of β-Catenin Signaling in Hormone Refractory PC-3 Prostate Cancer Cells. Nutr. Cancer 2012, 64, 153–162.
- Kumi-Diaka, J.; Saddler-Shawnette, S.; Aller, A.; Brown, J. Cancer Cell International Potential Mechanism of Phytochemical-Induced Apoptosis in Human Prostate Adenocarcinoma Cells: Therapeutic Synergy in Genistein and β-Lapachone Combination Treatment. Cancer Cell Int. 2004, 4, 5.
- Li, Y.; Sarkar, F.H. Gene Expression Profiles of Genistein-Treated PC3 Prostate Cancer Cells. J. Nutr. 2002, 132, 3623–3631.
- Kazi, A.; Daniel, K.G.; Smith, D.M.; Kumar, N.B.; Dou, Q.P. Inhibition of the Proteasome Activity, a Novel Mechanism Associated with the Tumor Cell Apoptosis-Inducing Ability of Genistein. Biochem. Pharmacol. 2003, 66, 965–976.
- Chen, Z.; Hagler, J.; Palombella, V.J.; Melandri, F.; Scherer, D.; Ballard, D.; Maniatis, T. Signal-Induced Site-Specific Phosphorylation Targets I Kappa B Alpha to the Ubiquitin-Proteasome Pathway. Genes. Dev. 1995, 9, 1586–1597.
- Chen, Z.J.; Parent, L.; Maniatis, T. Site-Specific Phosphorylation of IκBα by a Novel Ubiquitination-Dependent Protein Kinase Activity. Cell 1996, 84, 853–862.
- Traenckner, E.B.; Pahl, H.L.; Henkel, T.; Schmidt, K.N.; Wilk, S.; Baeuerle, P.A. Phosphorylation of Human I Kappa B-Alpha on Serines 32 and 36 Controls I Kappa B-Alpha Proteolysis and NF-Kappa B Activation in Response to Diverse Stimuli. EMBO J. 1995, 14, 2876–2883.
- Davis, J.N.; Kucuk, O.; Sarkar, F.H. Genistein Inhibits NF-KB Activation in Prostate Cancer Cells. Nutr. Cancer 1999, 35, 167–174.
- Kane, L.P.; Shapiro, V.S.; Stokoe, D.; Weiss, A. Induction of NF-ΚB by the Akt/PKB Kinase. Curr. Biol. 1999, 9, 601-S1.
- Singletary, K.; Milner, J. Diet, Autophagy, and Cancer: A Review. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1596–1610.
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-MTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Mol. Cell Biol. 2012, 32, 2–11.
- Tepper, C.G.; Vinall, R.L.; Wee, C.B.; Xue, L.; Shi, X.-B.; Burich, R.; Mack, P.C.; de Vere White, R.W. GCP-Mediated Growth Inhibition and Apoptosis of Prostate Cancer Cells via Androgen Receptor-Dependent and -Independent Mechanisms. Prostate 2007, 67, 521–535.
- Li, M.; Zhang, Z.; Hill, D.L.; Chen, X.; Wang, H.; Zhang, R. Genistein, a Dietary Isoflavone, Down-Regulates the MDM2 Oncogene at Both Transcriptional and Posttranslational Levels. Cancer Res. 2005, 65, 8200–8208.
- Choi, Y.H.; Lee, W.H.; Park, K.-Y.; Zhang, L. P53-Independent Induction of P21 (WAF1/CIP1), Reduction of Cyclin B1 and G2/M Arrest by the Isoflavone Genistein in Human Prostate Carcinoma Cells. Jpn. J. Cancer Res. 2000, 91, 164–173.
- Agarwal, R. Cell Signaling and Regulators of Cell Cycle as Molecular Targets for Prostate Cancer Prevention by Dietary Agents. Biochem. Pharmacol. 2000, 60, 1051–1059.
- Oki, T.; Sowa, Y.; Hirose, T.; Takagaki, N.; Horinaka, M.; Nakanishi, R.; Yasuda, C.; Yoshida, T.; Kanazawa, M.; Satomi, Y.; et al. Genistein Induces Gadd45 Gene and G2/M Cell Cycle Arrest in the DU145 Human Prostate Cancer Cell Line. FEBS Lett. 2004, 577, 55–59.
- Wang, B.F.; Wang, J.S.; Lu, J.F.; Kao, T.H.; Chen, B.H. Antiproliferation Effect and Mechanism of Prostate Cancer Cell Lines as Affected by Isoflavones from Soybean Cake. J. Agric. Food Chem. 2009, 57, 2221–2232.
More
