Head and neck squamous cell cancer (HNSCC) is one of the ten most common malignant neoplasms, characterized by an aggressive course, high recurrence rate, poor response to treatment, and low survival rate. This creates the need for a deeper understanding of the mechanisms of the pathogenesis of this cancer. The tumor microenvironment (TME) of HNSCC consists of stromal and immune cells, blood and lymphatic vessels, and extracellular matrix. It is known that HNSCC is characterized by complex relationships between cancer cells and TME components. TME components and their dynamic interactions with cancer cells enhance tumor adaptation to the environment, which provides the highly aggressive potential of HNSCC and resistance to antitumor therapy. Basic research aimed at studying the role of TME components in HNSCC carcinogenesis may serve as a key to the discovery of both new biomarkers–predictors of prognosis and targets for new antitumor drugs. This review article focuses on the role and interaction with cancer of TME components such as newly formed vessels, cancer-associated fibroblasts, and extracellular matrix.
- head and neck squamous cell carcinoma
- HNSCC
- tumor microenvironments
- vascular component
- extracellular matrix
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide, causing more than 660,000 new cases and 325,000 deaths annually [1,2]. Modifiable risk factors for this pathology include tobacco use in one form or another, alcoholic beverages, human papillomavirus (HPV) infection (more commonly associated with oropharyngeal cancer), and Epstein–Barr virus (EBV) infection (especially for nasopharyngeal cancer). People in some countries eat areca nut, which also increases the risk of HNSCC. Previously, HNSCC was classified according to tumor location (tumor of the oral or nasal cavity, oropharynx, nasopharynx, larynx, or hypopharynx), TNM (tumor, nodus, and metastasis) stage, and histology, but the recognition of molecular genetic profiles now allows it to be more accurately classified into individual subtypes [3,4]. Today, the updated TNM classification (eighth edition, 2018), which has several differences from the previous seventh edition of 2010, should be used for staging this nosology. The main changes in HNSCC staging include adding depth of invasion for oral cavity tumors, introducing a pathomorphologic and clinical staging system for high-risk oropharyngeal tumors associated with papillomavirus infection (HPV+), and considering tumor extension beyond the lymph node capsule in high-risk HPV-negative oropharyngeal tumors and head and neck squamous cell carcinoma in other localizations, excluding
nasopharyngeal cancer. Assessing the depth of invasion of oral cancer has prognostic value: deeper tumors show an increased risk of metastasis to lymph nodes and a decreased overall survival rate [5]. Extranodal extension, in turn, also serves as an unfavorable prognostic factor for HNSCC, with the exception of HPV+-related tumors [6]. A separate chapter in the eighth edition is devoted to nasopharyngeal cancer. The main changes are the inclusion of a T0 category for patients with metastatic cervical lymph nodes, EBV-positive patients with an unknown primary focus, and changes in the definition of regional lymph nodes. Unlike other localizations of head and neck cancer, for which surgery plays an important role in primary treatment, squamous cell cancer of the nasopharynx is primarily treated with radiation therapy, with or without chemotherapy. For this reason, pathologic classification is irrelevant in this disease of the nasopharyngeal region [7]. In 2022, the fifth edition of the World Health Organization (WHO) classification of head and neck tumors was published, focusing on the distinctive molecular genetic characteristics of head and neck tumors [8]. The delineation of the distinct molecular genetic signatures of HNSCC allows for a significant increase in diagnostic accuracy and also has prognostic value, which, in turn, leads to a personalized treatment approach for each patient. A recent review [9] details the results of the updated WHO classification for squamous HNSCC.
There are many approaches to the treatment of HNSCC, but none of them are effective enough. In the early stages of the tumor (Stages I and II), surgical removal and radiation therapy are highly effective, but 70% of patients are diagnosed with later stages of the disease—III or IV, where the effectiveness of these methods of therapy sharply decreases [10,11]. The ineffectiveness of therapy in a number of patients dictates the need for a deeper understanding of the mechanisms of the pathogenesis of head and neck tumors. To solve this problem, many studies have been conducted to study the properties and relationships between tumor cells and the microenvironment. HNSCC is characterized by complex relationships between stromal, epithelial, and immune cells in the tumor microenvironment (TME) [12–14]. The HNSCC TME includes various cells, both stromal and immune, as well as the extracellular matrix (ECM), blood, and lymphatic vessels [13,15]. Recently, a number of authors have emphasized the role of stromal cells in the induction and maintenance of tumors [16–18] and have also shown the relationship stromal cells have with the resistance of tumor cells to therapy [19]. We previously described, in detail, the role of immune cells in the development of head and neck tumors [20]. The current review discusses components of the tumor microenvironment such as neovasculature, hypoxia, cancer-associated fibroblasts (CAFs), and the ECM. By combining the accumulated knowledge of researchers around the world in this field, we hope to provide a better understanding of the interaction between HNSCC and its microenvironment to contribute to the discovery of novel biomarkers and targets for antitumor agents.
- Vascular Component of the HNSCC TME
The formation of new vessels, or angiogenesis, is one of the signs of the tumor process [21] and is critical both for the growth of the primary tumor and for the development of distant metastases [22]. Tumor angiogenesis and neovascularization are structurally and functionally different from healthy angiogenesis, with tumor vessels having blunt ends and poor perfusion [23]. Tumor endothelial cells have numerous ruptures, which contribute not only to blood leakage but also to the formation of blood clots and tissue swelling [24]. Various factors are involved in the formation of blood vessels in the TME, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), interleukin-8 (IL-8), delta-like ligand 4 (Dll4), and transforming growth factor families (such as TGF-β) [25,26] (Figure 1).
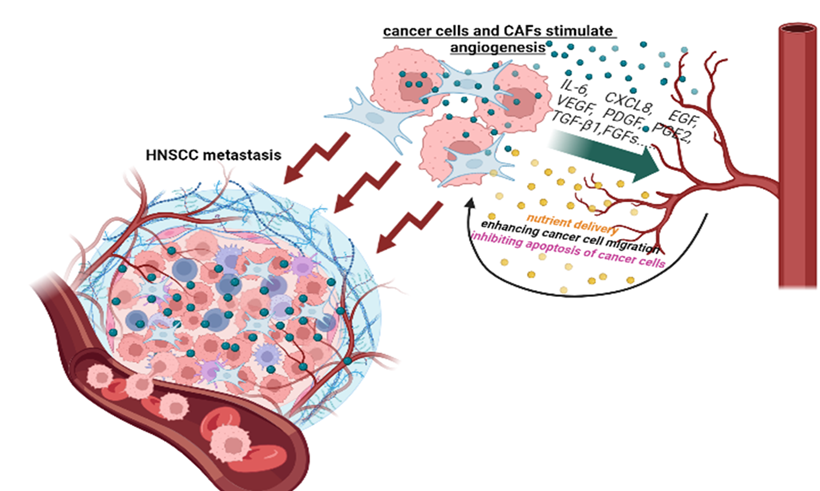
Figure 1. Metastasis of HNSCC as a result of the dynamic interaction of the tumor with blood vessels.
is a hypoxia-dependent gene and a key factor in tumor vascularization [27] that plays a crucial role in the regulation of blood vessel formation and maintenance. It belongs to the PDGF superfamily, which also includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and the placental growth factor (PlGF) [28,29]. Each member of the family has its own sphere of action. The most well studied is VEGF-A, which induces angiogenesis and is involved in various physiological and pathological processes, including the growth of malignant neoplasms. VEGF-A initiates the proliferation, migration, and tube formation of endothelium. In already formed vessels, it increases the permeability of the wall, allowing proteins, growth factors, and immune cells to penetrate tissues [30]. The functional potential of VEGF-B in cancer is less pronounced: it can enhance blood vessel growth, improve tissue perfusion, and protect against tissue damage under conditions of severe hypoxia [31]. VEGF-B can also interact with co-receptors called neuropilins (NRP-1 and NRP-2). VEGF-C and VEGF-D are associated with the process of lymphangiogenesis, the formation of lymphatic vessels. Thus, the overexpression of these ligands will promote the metastasis of cancer cells to lymph nodes. The last member of the VEGF family is PlGF, which becomes active in tumors under hypoxic conditions. It is also capable of tumor tissue and activating macrophages, which, in turn, secrete pro-inflammatory and pro-angiogenic cytokines such as IL-1 and TNF-α [32]. The VEGF family of ligands plays its role through the cell surface receptor tyrosine kinases, VGFR-1, VGFR-2, and VGFR-3 [33,34]. In addition, as mentioned above, VEGF interacts with NRP-1 and NRP-2 [35–37], which both enhance the association between VEGF and its receptors, increasing their biological activity. VEGF induces the proliferation, differentiation, and migration of vascular endothelial cells [38,39]; increases capillary permeability [40]; and increases endothelial cell survival by preventing apoptosis [41,42]. In turn, VEGF secreted by endothelial cells can enhance the migration of tumor cells [43], protect them from apoptosis, and prevent anoikis through the activation of PI3K/AKT in HNSCC cancer stem cells [44]. In addition to all of these functions, VEGF has been shown in a number of preclinical studies to contribute to immune suppression. This immunosuppression can occur in several ways. Firstly, VEGF, by binding to VEGFR1 on stem cells of myeloid origin, prevents their differentiation into mature immune cells. Secondly, it induces the expression of the programmed ligand PD-L1 on antigen-presenting cells, which leads to a decrease in T cell activation [45,46].
VEGF expression is influenced by various factors in the TME, including hypoxia, growth factors, cytokines, and transcription factors. For example, enhanced tumor vascularization is provided by the dynamic , which actively secrete pro-angiogenic factors [47–49]. Thus, TME macrophages, especially under hypoxic conditions, secrete TGF-β/-α, VEGF, IL-1, IL-6, and IL-8; these factors act as inducers of angiogenesis in HNSCC. For example, IL-8, IL-6, and EGF induce the phosphorylation of STAT3 and ERK in endothelial cells, which increases their survival and proliferation [50]. TGF-β, which is produced by many TME cells (CAFs, T regulatory lymphocytes, etc.) and is found in high percentages in HNSCC, also increases angiogenesis [51]. The high expression of angiogenic factors correlates with more advanced disease, resistance to conventional cytotoxic agents, and poor prognosis [22,52,53]. At least 90% of HNSCCs have increased expression of angiogenic factors such as VEGF. The overexpression of VEGF is associated with aggressive disease and poor outcomes in HNSCС [54,55]. In addition, the increased production of VEGF by tumor cells is associated with lymph node metastasis [56]. Aggarwal et al. [57] reported that serum VEGF levels were significantly higher in patients with oral squamous cell cancer and that these expressions were directly correlated with clinical stage evolvement and neck lymph node involvement. The interaction between HNSCC cells and endothelial cells triggers MAPK and Notch signaling, thereby promoting and enhancing tumor angiogenesis [58]. In a meta-analysis of 12 studies including 1002 patients with cancer of the oral cavity (70.8% of patients), pharynx (15.2%), and larynx (14%), VEGF expression was assessed, and its positivity was associated with a twofold increase in mortality after 2 years [22]. In addition, the overexpression of TGF-β1 found in HNSCC ultimately leads to tumor growth and metastasis by facilitating angiogenesis [59].
3. Role of the ECM in HNSCC
As discussed above, HNSCC is composed of a collection of malignant epithelial cells and TME cells (e.g., fibroblasts, vascular cells, immune cells) that secrete ECM proteins and numerous signaling molecules. Although the initiating genomic changes associated with HNSCC occur in epithelial cells, the changes that occur in the TME facilitate tumor progression and provide various adaptation mechanisms when exposed to damaging factors from the outside (anticancer drugs, radiation therapy) [158]. The ECM is involved in
virtually all stages of carcinogenesis, including cancer cell proliferation and invasion, angio- and lymphangiogenesis, the evasion of immune responses, the creation of an immunosuppressive environment, metastasis, and resistance to anti-tumor therapies [159]. The ECM is one of the main components of intercellular and intertissue interactions, providing signaling activity. It has a supporting and shaping function, providing the characteristic shape and size of organs and tissues [160,161]. The ЕCM is composed of fibrillar and non-fibrillar collagens, elastic fibers, and glycosaminoglycans (GAGs). . In tumor tissue undergoes changes—its composition, organization, and mechanical properties are altered. The altered composition of the ECM of tumors affects cancer cells, ensures cell insensitivity to growth inhibitors, promotes angiogenesis, and protects against antitumor agents [158]. Many solid tumors are characterized by an abundance of various ECM molecules, such as fibrillar collagens, fibronectin, elastin, laminins, and hyaluronic acid, with the predominance of one or another component depending on the type of cancer [162–164]. Recently, the ECM has been assigned one of the main roles in the oncogenesis, metastasis, and progression of malignant neoplasms [165]. An altered ECM composition is characteristic of most solid tumors. At the same time, the predominance of one or another ECM component depends on the anatomical location of the tumor, the causative factors of its origin, and the stage of the disease. [166]. In addition, tumor ECM is biochemically distinct from healthy tissue. Tumor stroma is usually stiffer than normal tissue stroma (~400 Pa versus 150 Pa, respectively) [167,168]. At the same time, the increase in stiffness and the remodeling of the ECM begin to be traced at the stage of precancerous changes, which, in turn, also contribute to the malignant transformation of cells. The dynamic interaction during carcinogenesis between tumor cells, microenvironmental cells, and the ECM leads to aberrant mechanotransduction and further malignant transformation [169].
The ECM of HNSCC is a collection of molecules with a variety of cytokines, intermediate metabolites, nutrients, hormones, and chemokines secreted by tumor and TME cells [170]. The ECM promotes the adhesion and migration of cancer cells, which causes tumor progression and metastasis (). Given its ability to bind secreted factors, the ECM is considered a functional bioregulatory platform on which the processes of carcinogenesis can take place [171]. Because HNSCC represents a diverse and complex set of diseases, it exhibits a high level of ECM heterogeneity. Indeed, malignancies arising in histologically distinct mucosal squamous epithelia such as the oral cavity, larynx, hypopharynx, and oropharynx may exhibit distinct stromal features. In oral squamous cell carcinoma, single-cell mRNA profiling has revealed a prominent stromal compartment with large numbers of matrix-producing CAFs in the majority of tumors examined [172]. The active production of ECM components by CAFs leads to increased tumor stiffness, which activates oncogenic intracellular signaling pathways, such as the β-catenin, Akt, PI3K, and focal adhesion kinase (FAK) pathways, and suppresses tumor suppressor genes such as phosphatase and tensin homolog [173,174].
In HNSCC, signaling between malignant epithelial cells and stromal cells causes increased activity in ECM components that control carcinoma cell migration, modulate the cytokine milieu, and promote immune evasion in tumors [170]. During HNSCC carcinogenesis, changes also occur in the ECM: its composition and density are altered. In the early stages of HNSCC progression, nascent transformed epithelial cells induce a wound-healing-like response marked by the activation of stromal fibroblasts and the strong accumulation of ECM and ECM-related proteins collectively known as matrisomes [175]. CAFs are the main matrix-producing cells of the stroma. In a proteomic analysis of the ECM produced by CAFs isolated from HNSCC, collagens I, III, VI, and XII; fibronectin; tenascin C; were among the major core components [176]. Proteins associated with the ECM deposited by these CAFs include galectin-1, annexin A2, the ECM regulator SERPINH1, tissue transglutaminase (TG2), HTRA1, and lysyl
oxidase homolog 2 (LOXL2), while secreted factors associated with the ECM included insulin-like growth factor 2, several alarmins, Wnt5A, and FGF [170].
As mentioned above, stromal cells (to a greater extent) and cancer cells participate in the production of ECM. Reciprocal epithelial–mesenchymal interactions at the tumor–stroma interface enhance the expression of cancer cell matrisomal proteins and their incorporation into the ECM. To demonstrate the important role of these components in tumor progression, a proteomic study of pancreatic ductal adenocarcinoma was conducted, which showed that high levels of tumor-cell-derived matrisomal proteins correlate with poor patient survival [177].
Let us consider changes in some ECM components of HNSCC. As with other types of cancer, with HNSCC, there is a thickening of the ECM, in which collagen plays a significant role. During tumor growth, increased interstitial collagen deposition is accompanied by fiber reorganization and enzymatic cross-linking (lysyl oxidases and lysyl hydroxylases), which correlates with increased tumor invasive potential and poor patient survival [178,179]. To classify changes in collagen arrangement that accompany carcinoma progression, distinct patterns of fibrillar collagen organization, called “tumor-associated collagen signatures,” have been defined [180]. Also, collagen in the HNSCC ECM activates the tyrosine kinase DDR1 receptor in tumor epithelial cells, which triggers pro-tumor activity. Moreover, increased DDR1 expression is associated with worse overall survival [181]. DDRs (discoidin domain receptors) can spontaneously bind to collagen and are not regulated by intracellular or extracellular signals [182,183]. The issue of DDR expression in various cancers remains controversial today, but there is evidence of increased DDR expression in cancer. For example, the overexpression of DDR1 has been observed in HNSCC [184]. Lysyl oxidase-like 2 (LOXL2) is a member of the lysyl oxidase (LOX) family of secretory enzymes, which are lysine deaminases that cross-link ECM proteins such as collagen [185,186]. Increased levels of LOXL2 have been found in HNSCC tissue [187]. The increased expression of LOXL2 mRNA has also been detected in metastatic lesions of HNSCC [188].
Let us review the major glycoproteins of the ECM of HNSCC. Fibronectin (the major glycoprotein of the ECM) is significantly overexpressed in patients with this type of cancer and has been obviously correlated with higher pathological stages and poor prognosis [180,189]. The downregulation of this glycoprotein suppresses the proliferation, migration, and invasion of HNSCC cells and inhibits macrophage M2 polarization in vitro [189]. Another HNSCC ECM glycoprotein, tenascin C, which is involved in the modulation of the immune response in many diseases [190], is also subject to changes. Even in the last century, a number of studies have described the role of tenascin in carcinogenesis: it induces cell migration [191], angiogenesis [192], and the expression of MMPs [193], which themselves are involved in promoting tumor growth and invasion [194]. Tenascin C has also been shown to promote tumor progression in a carcinogen-induced immunocompetent mouse model of OSCC by stimulating the formation of an immunosuppressive stroma [195]. Laminin expression is also upregulated in HNSCC. Laminin-5 hyperexpression is associated with high rates of HNSCC budding, suggesting that it is associated with the establishment of an invasive phenotype [196].
Integrins are transmembrane heterodimers consisting of α- and β-subunits. Integrins can bind collagen [197] and bind to various proteins, such as fibronectin, fibrinogen, laminin, and vitronectin [198]. Integrins also connect the ECM to the intracellular actin cytoskeleton. In addition, integrins provide the process of mechanotransduction: integrins perceive the mechanical force of the ECM and then transmit signals to intracellular proteins, such as tyrosine kinases FAK and Src. The activation of αvβ3 integrin correlates with poor prognosis for patients with OSCC [199,200]. Integrin α5 (ITGA5) promotes the proliferation, migration, and invasion of HNSCC cells by regulating the activation of the PI3K/AKT signaling pathway, and increased expression is associated with poor prognosis [201]. The overexpression of integrin αvβ6 is observed in HNSCC and correlates with invasive potential and progression [199,202].
Perlecan is a heparin sulfate proteoglycan that has five domains and is one of the main components of the ECM, participating in cell proliferation and differentiation (through interaction with integrins) [203]. It plays an important role in lipid metabolism, inflammation, wound healing, thrombosis, and cancer angiogenesis [204]. In HNSCC, perlecan promotes tumor cell growth, chemoresistance, migration, and invasion, mainly by regulating heparin-binding growth factors such as FGF-2, VEGF-A, and Hedgehog (Hh) [159,205]. Periostin is another important proteoglycan of tumor ECM. According to one study [132], the overexpression of periostin can also be observed in HNSCC, which is associated with tumor proliferation and metastasis.
The ability of tumor cells and TME cells to synthesize ECM components critically influences tumor progression [206]. Understanding the nature of heterotypic interactions between tumor cells, the ECM, and CAFs in the TME will provide insight into the mechanisms underlying tumor progression and metastasis and identify new targets for antitumor agents. The densified structure of the ECM, observed in many types of cancer, determines tumor progression both by creating barriers to the entry of therapeutic agents and by creating certain conditions in the tumor tissue itself. Today, it is known that the densified structure of the ECM observed in various types of cancer leads to a loss of sensitivity to anticancer drugs [207] and radiation therapy [208]. In addition to creating a “protective shield” effect that is difficult for antitumor agents to overcome [209,210], dense ЕCM compresses blood vessels, which also prevents drugs from reaching tumor cells. The compression of blood vessels by dense ECM leads to local hypoxia [211,212], which, in turn, activates antiapoptotic pathways and stimulates neoangiogenesis [213]. Immune cells migrating toward tumor cells along a cytokine concentration gradient cannot reach the tumor because they will encounter a dense ECM. Thus, ECM density regulates the process of the infiltration of immune cells into tumor tissue [210,214]. Hypoxia and metabolic stress, which are a consequence of high ECM density, lead to an increase in the content of immunosuppressive factors IL-10, CCL18, CCL22, TGF-b, prostaglandin-E2, and VEGF-A [213,215,216]. In this case, TGF-b attracts T-reg cells into the tumor [217] and acts as an M-2 polarizer for macrophages [218]. Dense ECM induces the transition of tumor tissue cells into cancer stem cells (CSCs), which, in turn, actively proliferate in hypoxic environments. In addition, a number of studies have demonstrated the resistance of CSCs to anticancer drugs [169,219,220]. . The increased deposition of a number of proteoglycans and collagens leads to ECM remodeling. Remodeled ECM causes disruption in cell polarity and enhances growth factor transport, causing biochemical and biomechanical changes. These changes ultimately contribute to the metastatic cascade: cell migration into the interstitial matrix and then into the vasculature is stimulated [159,221].
The ECM (together with the basement membrane) is a barrier that tumor cells must overcome on the “pathway to vascular invasion” [222,223]. In this case, basal membrane disruption is defined as a critical event of tumor invasion that marks the beginning of the metastatic cascade [224,225]. The surface of HNSCC cancer cells is characterized by the presence of invadopodia, which play an important role in the process of tumor invasion. Invadopodia mediate tumor dissemination by degrading ECM-restrictive proteins with matrix MMPs [102]. MMPs are members of a family of calcium- and zinc-dependent endopeptidases that degrade other components of the ECM and thus ensure its constant renewal [226]. In total, about 24 members of the MMP family have been identified in humans. In addition to the above-mentioned function, MMPs destroy the basal membrane and capillary wall and stimulate neoangiogenesis; therefore, these enzymes have a key role in the progression of HNSCC [227–229]. A number of studies have demonstrated an increase in MMPs in HNSCC [230,231]. Interestingly, the level of MMP elevation depends on the anatomical localization of squamous cell cancer in the head and neck region.. For example, MMP1 and MMP10 are highly expressed in OSCC [232] and MMP3 expression is elevated in squamous cell carcinoma of the tongue [233]. The activity of MMP14, MMP2, and MMP9 in the ECM is significantly increased in HNSCC cell lines with high metastatic
potential, as well as in samples from patients with oral cancer with lymph node involvement [234,235]. MMP14 plays a key role in the early stages of tumor invasion and cancer progression. MMP14 is concentrated on the surface of cancer cell invadopodia and destroys a number of VSMC components: collagen types I, II, and III; fibronectin; tenascin; and perlecan. A recent study [236] demonstrated an association between MMP14 levels and the extranodal extension of OSCC. In addition, ECM metalloproteinase inducer (EMMPRIN, also known as CD147) is an additional factor involved in tumor invasion and metastasis. In hypopharyngeal squamous cell carcinoma, CD147 appears to mediate ECM degradation by stimulating the synthesis of MMPs and promote angiogenesis by stimulating VEGF expression [237]. One study [238] showed that MMP3 can be used as a potential biomarker for oral cancer progression. In addition, a recent study [231] found that MMP family expression correlates with the levels of infiltration of six immune cells, B cells, CD8 + T cells, CD4 + T cells, macrophages, neutrophils, and dendritic cells, suggesting that the MMP family may reflect immune status and serve as a prognostic sign in HNSCC. Kudo et al. demonstrated that the highly invasive HNSCC cell line MSCC-inv1 significantly overexpresses MMP19 [239]. MMPs have the molecular function of modulating a number of latent signaling proteins located in the ECM, including cytokines and growth factors such as resting TGF-β, which forms a complex with TGF-β-binding protein-1 in the ECM. Thus, TGF-β modulates MMP expression, resulting in a bidirectional regulatory loop that enhances TGF-β signaling and promotes cancer progression [240]. The MMP family can be used as therapeutic targets and prognostic biomarkers for HNSCC depending on their role in the disease. Some scientists have used sesamin extracted from the sesame oil of pepper bark to regulate MMP2, thereby inhibiting HNSCC migration and invasion [241]. Another team of researchers showed that mulberry leaf extract can inhibit the activity of MMP2 and MMP9 and inhibit the migration and invasion of HNSCC [242]. Considering the multicomponent nature of the ECM, not all possible roles of its components in the development and progression of HNSCC have been described to date. Increased knowledge about the role of ECM components in HNSCC will help guide the search for new diagnostic and treatment options.
Table 2.Role of ECM components in the progression of HNSCC.
|
Main Components of the HNSCC ECM |
What Are the Effects on HNSCC Progression? |
|
|
|
‑ Create migration pathways for cancer cells and affect their invadopodia [243], thereby promoting invasion and metastasis [244]. ‑ Promote PI3 kinase (PI3K) activity and induce invasion [245]. ‑ Induce resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) through the activation of mTOR via the AKT-independent pathway [246]. ‑ Promote the proliferation and invasion of cancer cells via the activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathway [247]. |
|
|
Elastin |
‑ A positive correlation has been observed between elastin degradation and the degree and stage of the disease [248]. |
|
|
Fibronectin |
‑ Takes part in the transformation of cancer cells into stem cancer cells; stimulates their growth, proliferation, and evasion of growth suppressors. ‑ Stimulates angiogenesis by increasing the content and transmission of VEGF-mediated signaling [249]. ‑ Provides resistance of cancer cells to anoikis through a mechanism involving fibronectin and αv/FAK integrin receptor signaling [250]. |
|
|
Laminins |
‑ Activate EGFR/MAPK signaling [251]. ‑ High expression is positively correlated with tumor=invasive potential and poor prognosis [252–254]. |
|
|
Hyaluronan |
‑ Participates in the activation of the migration, survival, and chemoresistance of cancer stem cells [255]. |
|
|
Tenascin-C |
‑ Stimulates proliferation, migration, angiogenesis, and metastasis [256]. ‑ Induces epithelial–mesenchymal transition. ‑ Induces and activates other signaling pathways in cancer cells such as JNK, Wnt, Notch, AKT/HIF1α, and TGF-β. ‑ Creates barriers for T-lymphocytes to enter the tumor. |
|
|
Integrins |
‑ Provide radioresistance [257]. ‑ Integrin αvβ3 is actively involved in tumor angiogenesis [258]. ‑ Overexpression of αv integrin subunit is associated with tumor invasion and metastasis [259]. ‑ Participate in HNSCC proliferation and invasion by activating the mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathway [247]. ‑ In nasopharyngeal carcinoma, the mRNA and protein expression levels of integrin αv also correlate with tumor size and lymph node spread [260]. |
|
|
MMPs |
MMP2 |
‑ Increased expression of MMP 2 in HNSCC is positively correlated with aggressive invasion, poor survival, and metastasis to lymph nodes. |
|
MMP9 |
‑ Destroys type IV collagen, a major component of the basal membrane, promoting invasion and metastasis [260]. ‑ Enhances the bioavailability of VEGF, thereby increasing the amount of VEGF in the tumor microenvironment [261]. ‑ MMP9 overexpression is associated with tumor recurrence, metastasis to lymph nodes, and the development of secondary primary cancer [262]. |
|
|
MMP13 |
‑ Increases the secretion of VEGF-A [263]. |
|
|
MMP14 |
‑ Overexpression is associated with extranodal spread and is associated with poor prognosis [236]. |
|
|
MMP17 |
‑ Assists in cell invasion. ‑ Stimulates movement of amoeboid cells in tumor tissue and invadopodia [264]. ‑ Induced by HIF1-⍺-mediated hypoxia and enhances metastasis [264]. |
|
|
MMP20 |
‑ Promotes EMT; facilitates migration of cancer cells across the basal membrane [265]. |
|
|
Perlecan |
‑ Involved in adhesion and migration of cancer cells. ‑ Overexpression is associated with resistance of HNSCC cancer cells to cisplatin [266]. |
|
4. Conclusions
. The TME is a key component of this cascade of events, as it is involved in phenotype changes (cancer cells acquire a stem cell phenotype, immune cells acquire a pro-tumor phenotype) and the modulation of the behavior of both tumor cells and all cellular components of tumor tissue. Analyzing the relationships between TME components, one gains an impression of some “vicious circles” that condition the aggressive potential of HNSCC. Understanding the peculiarities of heterotypic interactions between cancer cells, new vessels, CAF, ECM, and hypoxia will allow us to understand the mechanisms mediating rapid tumor progression and its resistance to antitumor drugs and radiation therapy. Basic research aimed at studying the role of TME components in HNSCC carcinogenesis may serve as a key to the discovery of both new biomarkers–predictors of prognosis and targets for new antitumor strategies.
