Isolated pancreatic metastases of renal cell carcinoma (IsPMRCC) are a rare manifestation of metastatic, clear-cell renal cell carcinoma (RCC) in which distant metastases occur exclusively in the pancreas. In addition to the main symptom of the isolated occurrence of pancreatic metastases, the entity surprises with additional clinical peculiarities: (a) the unusually long interval of about 9 years between the primary RCC and the onset of pancreatic metastases; (b) multiple pancreatic metastases occurring in 36% of cases; (c) favourable treatment outcomes with a 75% 5-year survival rate; and (d) volume and growth-rate dependent risk factors generally accepted to be relevant for overall survival in metastatic surgery are insignificant in isPMRCC.
- renal cell carcinoma
- isolated pancreatic metastases
- genetics
- epigenetics
1. Introduction
2. Genetic Characteristics and Peculiarities of the isPMRCC
2.1. Clear-Cell RCC Genome
The genome of the ccRCC was deciphered as early as 2013 [46][57]. It is characterized by the biallelic absence or functional inactivation of the VHL tumour suppressor gene localized at 3p25 and the frequent inactivation of chromatin-modifying genes, such as PBRM1, BAP1 and SETD2 [47][58] (Table 1). The protein encoded by the VHL gene (pVHL) mediates its tumour-suppressive effect by binding to and mediating the proteasomal degradation of the hypoxia-inducible factor HIFα [48][49][59,60]. Under physiological conditions, HIFα subunits are unstable and are regulated by cellular oxygen content [50][61]. The loss or inactivation of VHL with consecutive inactivation of pVHL, therefore, leads to the activation and enrichment of HIF despite normoxic conditions and irrespective of the cellular oxygen availability and triggers the subsequent up-regulation of numerous HIF target genes. The activation of these HIF target genes is crucial for the formation and progression of ccRCC due to their role in promoting angiogenesis, tumour cell survival, proliferation and progression. HIFα consists of the subunits 1α and 2α, both of which are involved in ccRCC initiation [49][51][60,62]. During further ccRCC progression, however, HIF1α expression (located at chromosome 14q23 [52][53][63,64]) is lost in 30–40% since it can act as a tumour suppressor during the progression of ccRCC [49][53][60,64]. However, HIF2α acts as an oncoprotein in ccRCC. Due to the behaviour of HIF, two forms of ccRCC can be distinguished: Those in which HIF1α and 2α are overexpressed, and those in which only HIF2α is overexpressed and which are associated with enhanced cell proliferation and unfavourable prognosis [49][60]. HIF2α-triggered target factors include VEGF-α [49][54][60,65], TGF α/EGFR [55][66], c-Myc [49][56][57][60,67,68], cyclin D1 [58][59][69,70], SLC7A5-mTorC1 [49][60][61][60,71,72], GLUT1 [62][63][73,74], antioxidant enzymes [64][75], mitochondrial biogenesis factors [65][76], GAS6/tyrosine kinase AXL [66][77] and CXCR4/SDF1 [67][78], which control critical biological activities such as tumour angiogenesis, cell-autonomous proliferation, increasing glycolysis, resistance to oxidative damage, endoplasmic reticulum stress and metastatic ability [49][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][60,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81]. Further frequently altered genes in ccRCC are chromatin-modifying genes: polybromo-1 (PBRM1), BRCA1 associated protein 1 (PAB1), SET domain containing 2 histone-lysine N-methytransferase (SETD2), located on the same 3p chromosomal region [71][72][73][82,83,84], and less frequently, lysine demethylase 5C (KDM5C) located on the X chromosome [74][85] and telomerase reverse transcriptase (TERT) promoter located on chromosome 5p [75][76][86,87]. The frequency of detectable VHL defects is estimated to be up to 90% [47][75][77][78][79][58,86,88,89,90]. In contrast, the incidence of the other altered driver genes is significantly lower: PBRM1 52.6–26.4%, SETD2 35–7.6%, BAP1 31–7.5%, KDM5C 16–3.8%, TERT 14–12.2% and mTor 13–5.7% [47][73][75][77][78][80][81][82][83][58,84,86,88,89,91,92,93,94]. It was soon recognised that these gene alterations are associated with a different tumour biology, and thus, have an influence on the course of the disease and the outcome [79][84][90,95]. PBRM1 is the most frequently mutated gene after VHL [73][81][84,92] and mutations acquired in this gene largely do not overlap with loss of function mutations in BAP1 [47][77][79][81][85][58,88,90,92,96]. PBRM1 mutations are associated with improved outcome in ccRCC [84][86][95,97] and do not correlate with decreased survival [77][88], whereas the absence of mutations of PBRM1 resulted in worse outcome [79][90]. KDM5C mutations have also been associated with improved clinical outcome in clinical reports [77][83][88,94]. In particular, the concurrent mutations of PBRM1 and KDM5C define a subgroup with increased angiogenesis associated with favourable prognosis, as Santos reports [84][95]. The similar effects of PBRM1 and KDM5C mutations on outcome are consistent with the observation that the vast majority of up- and downregulated genes after suppression of PBRM1 or KDM5C were shared [87][98]. Conversely, PAB1 mutations in ccRCC have proved to be a driver of aggressiveness and correlated with reduced outcome [47][73][77][78][79][81][88][89][90][91][58,84,88,89,90,92,99,100,101,102]. PAB1 mutations further tended to be associated with mTOR mutations [81][92]. TERT and TP53 were also identified as gene mutations associated with a poor prognosis [47][75][79][88][58,86,90,99]. However, these gene changes are generally relevant to the occurrence and course of RCC, but none of these changes can be considered specific to the occurrence of metastases, let alone isPMRCC.| Altered Genes | References | |
|---|---|---|
| Clear cell RCC | VHL Gen | [46][47][71][77][57,58,82,88] |
| Chromatin modifying genes: e.g., PBRM1, BAP1, SET2, KDM5C | [72][75]86[77][78],88[81],89[83][83,,92,94] | |
| Further driver genes: e.g., pTEN, TERT, p53 | [75][78][81][82][83][88][86,89,92,93,94,99] | |
| Metastatic RCC | Loss of 9p, 14q Number of somatic copy number variants in primary ↑ metastatic potential ↑: low ITH 1 and high SCNA 2 in primary |
[52][92][93][63,103,104] |
| isPMRCC | 9p loss missing Number of somatic copy number variants ↓ chromatin-modifying genes: PBRM1 ↑, BAP1 ↓, KDM5C ↑ High genetic stability, constrained evolutionary process |
[80][92][94][50,91,103] |
2.2. Genetic Profile of Metastatic ccRCC
For the question of possible genetic characteristics of the isPMRCC, studies that specifically investigated genetic alterations that control and influence the metastatic behaviour of the RCC are therefore more relevant. Such a study was conducted and presented for the first time in 2018 by Turajlic [92][103]. In this groundbreaking analysis of 575 primary and 335 metastatic biopsies across 100 patients with metastatic ccRCC, the authors were able to identify three genetic changes that shape the metastatic behaviour of the RCC: 1. the loss of 9p21.3 and less pronounced 14q31.2 are hallmark genomic alterations at the beginning of the metastasis process; 2. the metastasis potential of RCC is reduced by low intratumoural heterogeneity and a small proportion of somatic copy-number alterations; and 3. distinct patterns of metastasis are caused by punctuated and branched evolution (Table 1).2.3. Genetic Profile of isPMRCC
So far only three publications have been presented in which this particular problem is addressed [80][92][94][50,91,103]. On the one hand, this is an inevitable consequence of the extreme rarity of isPMRCC, but on the other hand, it is also due to the fact that techniques such as next-generation sequencing have only been developed and used in recent years [71][82] (Table 1).- (a)
-
In the already cited work of Turajlic [92][103], among the 100 patients, there were also three isPMRCC observations, whose genetic profile was analysed and presented in detail for the first time. The isPMRCC showed an independent genetic profile characterized by the absence of 9p loss and a significantly lower genome instability index: Despite a 15-year and 8-year interval between primary ccRCC and clinical manifestation of PM, only one additional driver mutation was observed in two cases (mTor and SETD2, respectively) and in the third case, even after 17 years, there was no additional driver event to prove.
- (b)
-
Based on the improved prognosis of multiorgan metastases of ccRCC with concurrent PM compared to cases without PM, as shown by Grassi [95][105], and since repeatedly confirmed [11][80][86][96][97][98][99][100][11,91,97,106,107,108,109,110], Singla and colleagues in 2020 focused on the question of genetic characteristics of PM in mRCC [80][91]. (Their study group included 31 patients, but only a subgroup of just 10 (32%) met the isPMRCC criteria. However, the larger group (68%) experienced PM with simultaneous extrapancreatic multiorgan metastases of the ccRCC, which needs to be considered when assessing the relevance of the results for the specific isPMRCC topic discussed here because the detailed differences in metastasis behaviour between the two groups (single organ vs. multi-organ metastases) and the very special clinic of the isPMRCC (9.5 years metastasis-free interval until occurrence of PM and 75% 5-year survival rate, Section 1) make some genetic/epigenetic differences at least possible). In their extensive, meritorious study, Singla and colleagues were able to document genetic changes associated with less aggressive disease pathways: a low frequency of copy number variants associated with aggressiveness, such as 9p, 14q and 4q loss [52][92][93][63,103,104]. Furthermore, the authors found a low rate of PAB1 (3%) and a high rate of PBRM1 defects (77%)—changes associated with a less aggressive disease course [85][101][96,111]. Similarly, no driver mutation could be detected in TERT, which is associated with an aggressive disease course in RCC [75][86]. In contrast, KDM5C—after VHL and PBRM1—was the third most common gene mutation in the studied material with a frequency of 24%. As already pointed out above (Section 2.1), the concurrent occurrence of PBRM1 and KDM5C mutations is again a sign of a favourable course [84][95]. The high frequency of KDM5C mutations differs from metastatic ccRCC without PM in two respects. On the one hand, the value of 24% is the highest reported frequency so far [73][77][78][81][84,88,89,92]. On the other hand, in non-isPMRCC studies, KDM5C was only the fifth most common mutation [47][73][77][78][81][83][58,84,88,89,92,94]. As a further important characteristic of PMRCC, these authors also stress the unusual genetic stability of tumour cells, as limited diversification was observed both in the primary tumours leading to PM and in the subsequent PM themselves. The authors concluded that tumours and metastases from patients with PM are consistent with a constrained evolutionary process.
- (c)
-
Finally, Lou presented in 2023 an isPMRCC [94][50] that showed in the next-generation sequencing, three gene mutations (VHL, PTEN, KDM5C), a low tumour mutation burden and a microsatellite stable status. The fact that of the chromatin-modifying factors, only KDM5C was mutated is striking, as it further confirms Singla’s result of an increased frequency of KDM5C mutations (Figure 1).
-
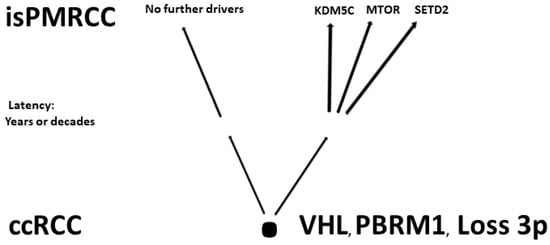 Figure 1.Involved genes in isPMRCC.
Figure 1.Involved genes in isPMRCC.
3. Conclusions
In isPMRCC, research in recent years has uncovered numerous genetic and epigenetic mechanisms that can explain the unusually protracted and favourable course and the specific response to drug therapy: e.g., high genetic stability, low frequency of copy number variants, a profile of chromatin modifying genes alterations associated with favourable course (PBRM1 ↑, PAB1 ↓) and affiliation to the angiogenetic subtype. However, the cause of the isolated occurrence of PM in isPMRCC is still unknown. The uniform long-term constant clinical course suggests at least that the phenomenon of isPMRCC is also based on uniform pathomechanisms. Therefore, genetic studies appear appropriate to clarify the mechanisms that cause the exclusive occurrence of pancreatic metastases and trigger their absence in all other organs. -
