1. Retinitis Pigmentosa
Use of electrophysiology in the diagnostic workup for Retinitis pigmentosa (RP) can be valuable in the early detection and characterization of disease. The full-field electroretinogram (ffERG) may detect the early characteristic photoreceptor degeneration, even before symptoms are noticeable for patients, making it useful for the diagnosis of RP
[1][36]. Full-field ERG recording enables the distinction between predominantly rod or cone system dysfunction (DA or LA protocols, respectively) and between generalized outer and inner retinal dysfunction. As seen in
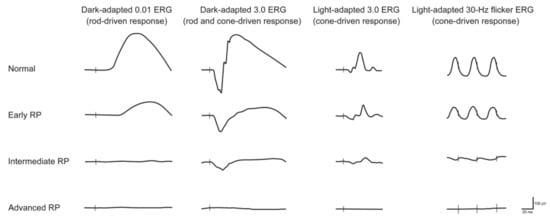
Figure 1, the ffERGs of RP patients characteristically include significantly decreased ERG amplitudes and increased latency compared to normal eyes in five ffERG protocols (DA 0.01, DA 10.0, OP, LA 3.0 and LA 3.0 30 Hz flicker)
[2][3][65,66]. The LA 30 Hz, which objectively assesses cone system sensitivity, also correlates strongly with reduced visual acuity in RP patients
[4][67]. These electrophysiologic findings correlate with clinical symptoms of nyctalopia and constriction of peripheral visual fields (rod photoreceptor dysfunction) and reduction in central vision (progressive cone photoreceptor dysfunction). Additionally, the location of the lesion affects the signal response to different extents. For example, the amplitudes of responses of rod, rod-cone, and cone
[5][68] of typical RP patients with peripheral lesions gradually extending to the central retina are lower than those of pericentral RP patients with lesions at the major temporal arcade on the retina that relatively spare the far periphery
[6][69].
Figure 1. Electroretiongram (ERG) recordings in early, intermediate and advanced stages of retinitis pigmentosa (RP). Vertical lines indicate the moment of stimulus flash. As the RP progresses, the amplitude of responses decreases, and the implicit time may increase. Cone dysfunction typically lags behind the onset of rod dysfunction. Eventually, the ERG—under both scotopic and photopic conditions—is extinguished (reused from Verbakel et al., 2009 with permission
[7][38]).
In RP patients without visual acuity deterioration, the N1 and N1P1 mfERG components showed preserved central retinal function but were severely reduced outside zone 2 of the retina
[8][70]. In RP, the mfERG delayed latency and decreased amplitude compared to controls has been shown to correspond with the regions of visual field losses
[9][71]. The decreased amplitude of the central segment of mfERGs and increased latency of mfERG in the central retina have significant correlations to the loss of visual acuity
[10][72]. Additionally, focal macular electroretinography amplitudes are reduced before the EZ thinning on OCT in the early stage of RP, which indicates that the focal macular ERG can be an earlier and reliable indicator of macular dysfunction
[11][73]. However, mfERGs, like PERGs, may not be recommended as a primary outcome measure in patients with advanced RP and nonrecordable ffERG because the response in all tested areas can be very low
[12][74]. On the electrooculogram (EOG), which measures the RPE potential in response to dark and light conditions, advanced RP is associated with a reduced light peak (Lp) to dark trough (Dt) ratio (i.e., Arden ratio)
[13][14][15][16][62,75,76,77].
The combination of mfERG and multifocal visually evoked potential technique (mfVEP) helps determine if there is an impact of regional retinal function on the visually evoked cortical activity, as VEPs are generated primarily in the visual cortex and may be affected by abnormalities along the visual pathway
[17][78]. As seen in
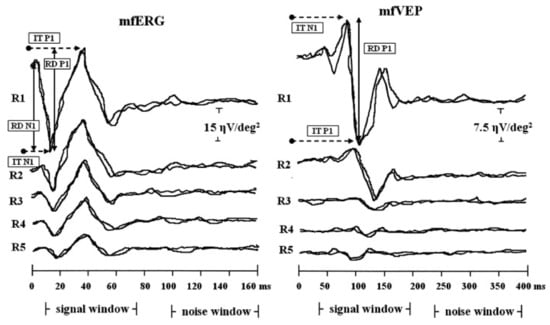
Figure 2, significant positive correlations were found between the mfERG response amplitude density (RAD) and the mfVEP RAD, as well as between the mfERG implicit time and the mfVEP implicit time, indicating that some of the mfERG responses were quantitatively correlated with mfVEP response components at corresponding retinal locations
[18][10]. The functional measures (mfERGs) in RP usually confirm the preserved central retinal function with severely reduced function outside of the central retina, which may not be reflected by ffERGs
[19][79].
Figure 2. Examples of multifocal electroretinogram (mfERG) and multifocal visually evoked potential (mfVEP) recorded in one representative retinitis pigmentosa study eye. MfERG and mfVEP local responses were averaged in five retinal areas located at various degrees of eccentricity from the fovea: 0–2.5 (R1), 2.5–5 (R2), 5–10 (R3), 10–15 (R4) and 15–20 (R5) degrees. IT, implicit time; RAD, response amplitude density (reused from Parisi et al., 2009 with permission
[18][10]).
Overall, electrophysiology testing may be a valuable tool in the early diagnosis and monitoring of RP, as ffERG can identify early characteristic photoreceptor degeneration, even before symptoms are noticeable for patients. This is useful in many cases of early RP and other IRDs in which the retina may appear normal despite clinical symptoms and visual field defects. Furthermore, mfERG and PERG allow an objective evaluation of residual cone function in retinitis pigmentosa patients, which can provide guidance for patient prognosis as it relates to progressive blindness and reduced vision-related quality of life
[4][67]. MfERG, when combined with other techniques such as OCT and VEP, can provide useful information in monitoring macular function in RP
[10][72]. Using a combination of structural imaging and electrophysiological investigations will likely provide better estimations of retinal function in RP patients.
2. Progressive Cone/Cone-Rod Dystrophy
Use of electrophysiology for cone dystrophies can be valuable in the early detection and monitoring of possible rod photoreceptor involvement. ffERG of pure cone dystrophies or early CORD shows normal rod responses with abnormal cone signals
[20][21][97,98]. Cone dystrophy shows normal, preserved rod functions and severe cone dysfunction in ffERG
[22][93]. ffERG can be particularly helpful in early stages when patients are asymptomatic with a normal fundus exam
[23][90]. ERGs on family members with the
GUCA1A mutation show reduced amplitudes in the 30 Hz flicker and photopic (cones) over scotopic (rod) response, as well as severely reduced bilateral P50 amplitudes on PERG
[23][24][90,99]. Other ERG findings of cone dystrophy include implicit time delay with the 30 Hz cone flicker responses, delayed a- and b-wave photopic response, as well as decreased amplitudes of both a- and b-waves
[23][25][90,100]. In CORDs, both rod and cone ERGs are abnormal with predominant cone abnormalities with delayed 30 Hz cone flicker ERG implicit time
[26][27][17,101].
Overall, electrophysiology testing can be a valuable tool in the early diagnosis and monitoring of progressive cone dystrophy. ERG changes precede the decline in subjective visual functions
[28][96], allowing for early detection. ERGs also can isolate signals of the two major photoreceptor types (rods and cones), which helps distinguish between the spectrum of diseases ranging from COD to CORD
[25][100].
3. Cone Dystrophy with Supernormal Rod Response
The ERGs of cone dystrophy with supernormal rod ERG are pathognomonic and diagnostic of the disorder. Like other cone dystrophy disorders, the light-adapted (LA) 3.0 and LA 3.0 30 Hz ffERGs show significant reduction and delay in those patients
[29][106]. PERG is always abnormal in the patients with supernormal rod ERG and can be undetectable in severe macular dysfunction, although some other cone and cone-rods dystrophies may have relatively preserved macular function and PERGs in early disease
[30][107]. Several pathognomonic findings of this disorder occur in the rod system: (1) In response to a very dim white flash (0.002 phot cd s/m
2), there is no detectable ERG response. (2) In response to a standard dark-adapted dim flash (DA 0.01), the ERG shows significant delay of implicit time and subnormal amplitude. (3) From 0.01 phot cd s/m
2, b-wave amplitude increases rapidly with small increases in stimulus intensity. (4) In response to DA 10.0 ERG, a-wave shows a normal slope (normal phototransduction) at the beginning, followed by a broadened shape with smaller slope (a late negative component)
[29][31][106,108].
Overall, electrophysiology testing is the primary tool in the diagnosis of CDSRR due to its pathognomonic and characteristic findings. To further work up this suspected diagnosis, genetic testing is necessary to confirm the mutations in either the KCNV2 or PDE6H genes.
4. Enhanced S-Cone Syndrome
Enhanced S-cone syndrome (ESCS) is a rare, slowly progressive retinal dystrophy characterized by nyctalopia from the first decade of life, reduced visual acuity, hyperopia, and no nystagmus
[32][33][109,110]. ESCS is an autosomal recessive disorder related to mutations in
NR2E3, which encodes a photoreceptor nuclear receptor transcription factor critical for rod photoreceptor development, leading to excess S (blue) cones at the expense of other photoreceptor subtypes
[34][111]. The disease is characterized by an abnormal ratio of S (blue) to L/M (red/green) cone function, the absence of rods, and progressive retinal degeneration
[35][112]. In addition to the variety of mutations in the NR2E3 gene that causes the autosomal recessive ESCS, autosomal dominant mutations in NR2E3 have been found to cause retinitis pigmentosa, although the distinct inheritance patterns and electrophysiological findings can help differentiate these conditions
[36][37][22,23].
On fundus examination, there are characteristic deep nummular clumped pigmentary lesions around the vascular arcades with possible yellow streaks, as well as macular retinoschisis and cystoid maculopathy
[38][113]. On OCT, there is a significantly thickened outer nuclear layer as well as macular abnormalities including foveomacular schisis and macular holes
[39][114].
The abnormality of ERGs in ESCS is also pathognomonic of the disorder. ISCEV-standard recordings of ffERG in an ESCS patient are shown in
Figure 3. ffERG dark-adapted 0.01, which is rod-specific, is almost always undetectable due to the absence of rods
[40][115]. DA 3.0 ERG (mixed rod and cone response) is delayed and usually reduced
[34][111]. The 30 Hz flicker ERG shows characteristically delayed implicit time and reduced amplitude
[41][116]. S-cone specific and ON-/OFF ERGs show supernormal, higher amplitudes with simplified waveforms and delayed peak, which is pathognomonic of the disorder
[42][117]. The pattern ERG, if detectable, usually shows a delayed and reduced P50 component
[42][43][117,118]. MfERG shows preservation of central responses but delayed and reduced responses in peripheral rings
[42][117].
Figure 3. ISCEV-standard rod-specific, maximal mixed rod and cone, 30 Hz flicker stimulus, and transient single-cone ERG waveforms are shown. The scales, with normal ranges for each condition, are listed in the bottom row (reused from Park et al., 2013 with permission
[44][119]).
Overall, electrophysiological testing is an invaluable tool in the diagnosis of ESCS, which shows pathognomonic ERG findings and identifies patients for targeted specific molecular screening to finally confirm the diagnosis
[32][109].
5. Bradyopsia
The ERG of bradyopsia shows diagnostic findings of the disorder but also requires testing of cone function under dark adaptation and understanding of impaired recovery after a bright flash for correct diagnosis, which needs a protocol in addition to the current ISCEV standard protocols
[31][108]. DA 0.01 (rod-specific) ERG and DA 3.0 ERG are normal or mildly reduced, while LA 3.0 ERG is subnormal. The pattern ERG and standard LA 3.0 30 Hz flicker ERG are undetectable in all patients with the
RGS9 mutation
[45][120]. Additionally, there are important findings of the disorder: (1) a scotopic (rod) red flash ERG under dark adaptation evokes normal rod and dark-adapted cone function (but severely abnormal cone function under photopic conditions); (2) dim flicker stimulus under dark adaptation reveals normal ERG response at the beginning of stimulation, which attenuates to undetectable response after 2 s; (3) DA 10.0 ERG reveals normal response to the first bright flash, and the subsequent responses to further flashes are attenuated, which takes up to 2 min to recover
[45][46][24,120]. Similarly, ERG in the mouse model lacking
RGS9 shows a normal response in the first flash with reduction in subsequent flashes and prolonged recovery
[47][48][25,122].
Overall, in the diagnosis of bradyopsia, electrophysiological testing is a valuable tool that can identify characteristic ERG findings. Furthermore, additional ERG protocols can characterize the functional deficiencies.
6. Bietti Crystalline Dystrophy
Electrophysiology testing is more useful in assessing the extent of the disease rather than as an initial diagnostic tool in Bietti crystalline dystrophy (BCD). The ffERG shows varying degrees of rod and cone dysfunction, ranging from normal to reduced or undetectable amplitudes of scotopic and photopic a- and b-wave responses
[49][50][51][129,130,131]. The ffERG implicit times are also prolonged in BCD, especially in scotopic ERG, which correlates with the clinical presentation of nyctalopia
[52][132]. The progression of the disease may follow a rod-cone dystrophy pattern according to ffERG findings. Some studies show the correlation between ffERG and disease severity, in which the patients with RPE atrophy and intraretinal crystals confined to the posterior pole show normal or mildly affected ffERG
[49][129]. The ffERG is more likely to be abnormal in the later course of the disease when the peripheral visual field is severely affected
[53][28], although the ffERG can also remain normal in some patients in later stages of the disease, even with severe RPE and choroid atrophy
[54][133].
In BCD, mfERG could be helpful in detecting focal regions of abnormal retinal function, especially in the phenotypes that predominantly affect the posterior pole with normal ffERG. Significant reductions in N1 and P1 amplitudes as well as delays in P1 (and to a lesser degree in N1) implicit times of mfERG can be seen in BCD patients
[52][55][132,134], with relatively preserved peripheral responses
[52][56][132,135]. Because a BCD patient with normal peripheral retina may have normal ffERG but abnormal mfERG
[57][136], mfERG is more sensitive than ffERG in identifying the defective areas of the retina, which can be helpful in the evaluation and follow-up of these patients
[52][132].
Overall, electrophysiological testing may not be critical in the initial diagnosis of BCD. However, it can be used to monitor the progression of retinal degeneration in BCD over time.
7. Late-Onset Retinal Degeneration (L-ORD)
Use of electrophysiology for L-ORD can be helpful in some cases to identify abnormalities in early disease
[58][59][60][137,138,142], which often shows a generalized rod-cone pattern of dysfunction that appears to be more progressive at later stages of the disease
[61][62][140,143]. In L-ORD patients, significant reduction of scotopic ERG is usually observed
[63][144], while the photopic ffERG is affected in the later stage
[62][143]. There is correlation between the decreased ffERG response to areas of drusenoid deposits and atrophy
[59][61][62][64][65][30,138,140,143,145], because ffERG can still be elicited from the unaffected retinal areas
[65][145].
In a study of L-ORD with ffERG, there was significant improvement in b-wave under DA 0.01 and a-wave under DA 3.0 after a prolonged dark adaptation of 16 h, suggesting an opportunity for treatment in patients with moderate to advanced L-ORD. It suggests the possibility of partial reversion of rod dysfunction
[65][145].
In L-ORD, pattern ERG shows that there is also severe macular involvement in addition to rod-cone dysfunction on ffERG
[60][142]. Therefore, a severe macular involvement can be detectable using PERG in L-ORD patients. MfERG shows reduced amplitudes in the areas of drusenoids, corresponding to areas of reduced sensitivity on microperimetry maps
[66][146]. In three L-ORD patients, EOG showed a normal light peak/dark trough (Arden) ratio, with two cases demonstrating abnormal dark trough amplitude and delayed light peak
[62][143].
Overall, electrophysiological testing may not be a critical diagnostic tool for L-ORD because ffERG can be normal in some patients, especially in early disease
[67][147]. However, electrophysiological testing may be the objective complementary tool to monitor the progression of retinal degeneration as well as to identify partial reversion of rod dysfunction with extended dark adaptation in L-ORD.
8. Fundus Albipunctatus
Use of electrophysiology for fundus albipunctatus can be valuable and reliable in diagnosis and help differentiate patients who may also additionally have cone dystrophies. Most patients with fundus albipunctatus have a nonprogressive rod dysfunction without retinal vessel attenuation, or pigment clumping in the retina, which is similar to retinitis functata albescens
in Section 2.9. However, other patients with fundus albipunctatus may have cone dystrophy, leading to loss of central visual acuity and reduced cone ERG amplitudes
[68][32]. Standard scotopic ffERG shows moderate to severe generalized rod system dysfunction. Scotopic dim flash ERGs can be undetectable or subnormal but can be normalized after prolonged dark adaptation. Scotopic bright flash ERG shows a reduced maximal response. The use of a red stimulus under dark adaptation and extended recordings in the dark-adapted state suggest dark-adapted cones as the probable source of the ERG signals. An improvement in the dark-adapted ERG response is considered a feature of Fundus albipunctatus because of the slow rate of regeneration of photopigment but can also be found in retinitis punctata albescens patients
[69][151]. Photopic responses may have subnormal amplitudes
[69][70][71][151,152,153].
Overall, electrophysiology testing, together with genetic testing, is the most reliable tool in the diagnosis of FA. Additional retinal imaging (fundus photographs, fundus autofluorescence, and OCT) can show characteristic findings and may aid with the diagnosis. Particularly in younger patients with characteristic features of fundus albipunctatus, cone and rod function can revert to normal after a prolonged period of dark adaptation.
9. Retinitis Punctata Albescens
Utilizing electrophysiology for retinitis punctata albescens can be helpful in the diagnosis of the disease. ffERG performed after standard dark adaptation shows moderate to severe generalized rod system dysfunction. The dark-adapted isolated rod ERG response is severely reduced or undetectable
[72][157]. MfERG may show a reduced cone response in the central region of the tested area with no improvement after 20–24 h of dark adaptation
[73][158].
Overall, electrophysiology testing can be helpful in distinguishing RPA from fundus albipunctatus, which most often shows stationary rod receptor dysfunction and recovery of cone and rod function after a prolonged period of dark adaptation that is not often seen in RPA. Genetic screening, as well as the presence of progressive loss of photoreceptor cell function, attenuated retinal vessels and pigmentary clumping, are also the important clinical manifestations of RPA in a diagnostic workup to exclude fundus albipunctatus.