Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Michail Koutentakis and Version 2 by Lindsay Dong.
Sodium–glucose cotransporter-2 (SGLT-2) inhibitors, also called gliflozins or flozins, are a class of drugs that have been increasingly used in the management of type 2 diabetes mellitus (T2DM) due to their glucose-lowering, cardiovascular (CV), and renal positive effects.
- SGLT-2 inhibitors (gliflozins)
- T2DM
- cardiovascular
- ketogenesis
1. Introduction
SGLT-2 (sodium–glucose cotransporter-2 (SGLT-2) inhibitors, often referred to as gliflozins or flozins, achieved a significant milestone in 2012 with approval by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) for the treatment of type 2 diabetes mellitus (T2DM) [1][2][3][1,2,3]. This moment marked a paradigm shift in addressing elevated blood glucose levels. Within this context, the triad of endorsed SGLT-2 inhibitors, dapagliflozin (DAPA), canagliflozin (CANA), and empagliflozin (EMPA), emerged as pivotal components in the management of hyperglycemia. By concurrently modulating renal glucose reabsorption and enhancing urinary glucose excretion, this trio presented a potent therapeutic avenue for ameliorating the impact of T2DM [4][5][6][7][4,5,6,7].
While initially pursued for cardiovascular safety validation, SGLT2 inhibitors defied expectations in cardiovascular outcome trials (CVOTs) like EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in T2DM Patients), CANVAS (Canagliflozin Cardiovascular Assessment Study), DECLARE-TIMI-58 (Dapagliflozin Effect on Cardiovascular Events−Thrombolysis In MI (Myocardial Infarction) 58), VERTIS CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular outcomes), and SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with T2DM and Moderate Renal Impairment Who Are at Cardiovascular Risk). These trials surprisingly unveiled SGLT2 inhibitors’ ability to significantly reduce major adverse cardiovascular events (MACEs) compared to placebos. Notably, the 2015 EMPA-REG OUTCOME trial first demonstrated this protective effect, showcasing a 14% MACE reduction, 34% lower all-cause mortality, and 35% fewer heart failure (HF) hospitalizations [8]. Subsequent studies, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in HF) and EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic HFrEF (Heart Failure with Reduced Ejection Fraction)), revealed SGLT2 inhibitors’ transformative potential in HF treatment for patients with a reduced EF (ejection fraction) <40%, regardless of T2DM, decreasing hospitalizations, and mortality [9][10][9,10]. The EMPEROR-Preserved trial (Empagliflozin Outcome Trial in Patients with Chronic HFpEF (Heart Failure with Preserved Ejection Fraction)) in 2021 extended these benefits to chronic HF patients, irrespective of EFs, emphasizing SGLT2 inhibitors’ expanding role in enhancing prognosis [11].
In addition to that, the discovery of gliflozins’ nephroprotective effect has greatly impacted clinical practice as well. Diabetes is associated with microvascular damage, often culminating in chronic kidney disease (CKD) for around 40% of patients. Investigated in CVOTs, gliflozins effectively mitigate declines in the glomerular filtration rate (GFR), delay microalbuminuria, and hinder proteinuria progression, favoring both diabetic and nondiabetic patients. Recent EMPA-KIDNEY (The Study of Heart and Kidney Protection With Empagliflozin) trial data show SGLT2 inhibitors’ efficacy in nephropathy, even with a diminished eGFR (estimated glomerular filtration rate). Consequently, SGLT2 inhibitors assume a pivotal role in reducing the progression to end-stage renal disease (ESRD) among patients with CKD [12].
Beyond the aforementioned effects, SGLT-2 inhibitors have a pro-ketogenic effect that has been associated with their potential to increase the production of ketone bodies, such as BHB (β-hydroxybutyrate) [13]. This pro-ketogenic effect holds promising implications for cardiovascular events such as stroke and HF, as well as for retarding the progression of chronic diseases, such as atherosclerosis and CKD [14][15][14,15].
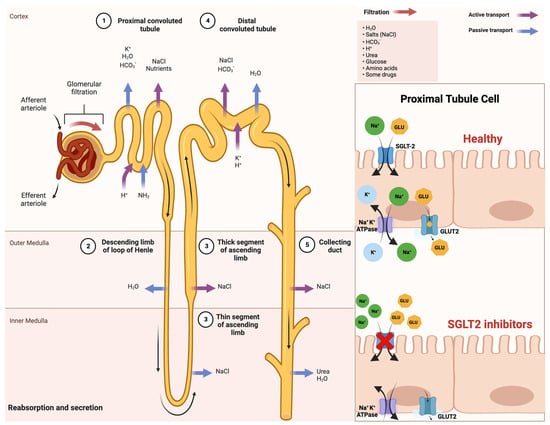
2. Mechanism of Action of SGLT-2 Inhibitors
Sodium–glucose cotransporters (SGLTs) are a class of transmembrane proteins that share a common transport mechanism in which extracellular sodium binding causes a gate to open and traps glucose from outside of the cell. The transporter then shifts, releasing sodium and glucose into the cytoplasm. The protein reverts to its initial conformation at the completion of the process. The two most common SGLTs are SGLT-1 (sodium–glucose cotransporter-1) and SGLT-2 (sodium–glucose cotransporter-2) [16][17][26,27]. The SGLT2 transporter is predominantly expressed in the epithelial cells of the renal proximal convoluted tubule. Although SGLT2 has a low affinity for glucose, it demonstrates a remarkable capacity for renal glucose reabsorption [18][28]. The process is primarily sodium-dependent, with SGLT2 and SGLT1 exhibiting ratios of 1:1 and 2:1, respectively. While SGLT1 is responsible for only approximately 10% of the tubular glucose reabsorption, SGLT2 handles the majority of the reabsorption. This capacity for reabsorbing filtered glucose in the kidneys is an extremely efficient energy conservation mechanism. Apart from the kidneys, SGLT2 expression has been detected in other organs such as the brain, liver, thyroid, muscles, and heart, while SGLT1 expression has been reported in the intestine, trachea, kidney, heart, brain, testes, and prostate. The high capacity of renal glucose reabsorption, especially by SGLT2 has led to the development of SGLT2 inhibitors as a treatment for T2DM [16][26]. Within this framework, SGLT-2 inhibitors function by obstructing the activity of the SGLT-2 protein, which is prominently present in the proximal convoluted tubules of the kidneys. This action effectively prevents the reabsorption of filtered glucose from the tubular lumen [19][29]. Their mechanism of action is based on the renal excretion of glucose, causing glucosuria, and is independent of insulin action, thus reducing hypoglycemia, weight gain, and liver disease [20][30]. An improved comprehension of SGLT2 inhibitors and their impact on insulin sensitivity holds promise for enhancing treatments for T2DM and metabolic disorders. This insight may also illuminate the intricate interplay between insulin sensitivity and glucose balance, which is vital for maintaining metabolic health. A schematic illustration that indicates the mechanism of action of SGLT-2 inhibitors in detail is shown in Figure 1.
Figure 1. Comprehensive schematical depiction of the mechanism of action of SGLT2 inhibitors. Abbreviations: SGLT2—sodium−glucose cotransporter 2; Na+—sodium cations; K+—potassium cations; H2O—oxygen hydride (water); HCO3−—bicarbonate ions; NaCl—sodium chloride (salt); H+—hydrogen ions; NH3—ammonia; GLU—glucose; ATPase—adenosine triphosphatase (enzyme); GLUT2—glucose transporter 2. Created with BioRender.com and accessed on 17 April 2021.
3. Synergism of SGLT-2 Inhibitors and Ketogenic Diet: Benefits and Risks
3.1. Positive Synergistic Effect of SGLT-2 Inhibitors and Ketogenic Diet: Benefits
Ketogenic diets and SGLT2 inhibitors are two highly promising therapeutic approaches for treating T2DM and other complications. In recent years, there has been a noticeable increase in interest in both of these strategies due to their potential synergistic health merits, such as weight reduction, improved insulin sensitivity, and decreased cardiovascular risk [21][22][23][59,60,61].
As previously noted, SGLT-2 inhibitors are a group of oral anti-diabetic medications that prevent the kidneys from reabsorbing glucose, enhance urine glucose excretion, and lower blood sugar levels [24][62]. On the other hand, the ketogenic diet, characterized by its low-carbohydrate and high-fat composition [25][26][63,64], consisting approximately of 70–80% fat, 10–20% protein, and 5–10% carbohydrates, induces ketosis, a metabolic state in which the body primarily uses fat for energy production by producing ketones rather than glucose [27][65]. Both approaches independently promote weight loss and improve glycemic control, which are crucial for managing these conditions [28][66].
In more detail, SGLT-2 inhibitors exert their weight loss effects through the increased urinary excretion of glucose, leading to a calorie deficit and a subsequent reduction in body weight. This weight loss is predominantly due to a decrease in adipose tissue, which is beneficial since excessive adipose tissue is primarily associated with insulin resistance and inflammation in T2DM [29][68].
To amplify these therapeutic benefits, the combined use of SGLT-2 inhibitors with a ketogenic diet may prove to be an innovative tactic. In fact, recent studies have suggested that combining SGLT-2 inhibitors with ketogenic diets may have additive effects on glucose control and other metabolic parameters [30][75]. Taking into consideration the fact that both interventions can lead to a decrease in HbA1c [31][32][76,77], body weight [29][33][68,78], and blood pressure [34][35][36][79,80,81], it seems reasonable to say that the combination of the two would result in even greater reductions in HbA1c, body weight, and blood pressure than either treatment alone, thereby offering a more effective approach to managing T2DM and obesity. On top of that, considering the anti-inflammatory effects of both treatments [37][38][82,83], their simultaneous use may lower the risk of chronic inflammation and associated CVD, bringing about superior outcomes.
Numerous assertions have been put forth regarding the neuroprotective advantages attributed to both ketogenic diets and SGLT-2 inhibitors, further emphasizing their potential impact on neurological health [38][39][40][83,84,85]. While the only well-established use of a ketogenic diet is for reducing seizures in pediatric epilepsy [41][86], its ability to produce ketone bodies that serve as alternative fuels for brain metabolism is key to maintaining mitochondrial function, ATP (the source of energy for use and storage at cellular level) production, and neuronal survival.
