Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Hammad Ullah and Version 2 by Peter Tang.
Marine cyanobacteria are an ancient group of photosynthetic microbes dating back to 3.5 million years ago. They are prolific producers of bioactive secondary metabolites.
- marine cyanobacteria
- climate change
- nitrogen fixation
1. Introduction
Cyanobacteria may be described as the pioneers of the planet Earth and are a one-of-a-kind gift of nature. The historical record dates the existence of cyanobacteria back to over 3.5 million years ago (MYA). They possess a number of natural qualities that make them suitable for several biotechnological applications [1][2][3][1,2,3]. Cyanobacteria (blue-green algae) are Gram-negative eubacteria. They are widely distributed throughout terrestrial, fresh water, wastewater, and marine habitats due to their immense morphological and physiological diversity [2][4][2,4]. Cyanobacteria come in a wide variety of morph types, such as species that are unicellular, surface-attached, as well as filamentous colony- and mat-forming. In the last few decades, the advent of ultrastructural and molecular methods has been evident as per the progress in taxonomic classification, the dominant tool for evaluating cyanobacterial diversity. These methods explain many yet unclear structures, adaptations, and phylogenetic relations within different clusters. The molecular methods rely on 16S rRNA gene sequencing as a standard, notably at the genus level. The 16S rRNA gene sequencing clusters closely correlate with traditional cyanobacterial taxa, which can be identified by various phenotypic characteristics [5][6][5,6]. Several species form significant symbiotic relationships with other micro- and macro-eukaryotes [7][8][7,8]. As life evolved on Earth, cyanobacteria played a pivotal role as the first organisms to engage in oxygenic photosynthesis [9]. In addition to their evolutionary niche, cyanobacteria have also played vital roles in the biogeochemical cycle of carbon (C) and nitrogen (N). An estimated 20–30% of the organic carbon present on Earth is derived from photosynthetic carbon fixation by cyanobacteria. Cyanobacteria also represent the primary N2-fixing microorganisms in marine environments [10][11][12][10,11,12].
Natural products have historically been used to inspire and develop new medications, and this remains one of the most successful methods for the discovery of small molecules for drug development [13]. Cyanobacteria are a unique source of small molecules, since they are thought to be one of the oldest forms of life on Earth and may thus constitute chemical factories with highly evolved secondary metabolite synthesis machinery. They have also been demonstrated to influence the biosynthesis of chemicals in marine invertebrates, such as sponges, ascidians, and shell-less mollusks, via endosymbiosis or diet-derived enrichment [13][14][15][16][13,14,15,16]. To date, more than 2000 secondary metabolites have been identified from marine cyanobacteria, especially Moorea, Lyngbya, and Okeania spp. [17][18][17,18]. Marine cyanobacteria have attracted the attention of many scientists in the fields of medicinal chemistry and pharmacology [19], as they demonstrate powerful biological activities such as antibacterial [20], antifungal [21], anticancer [22], and cytotoxic activities [23], as well as immunosuppression [24], anti-inflammatory [25], and antioxidant properties [26]. For instance, apratoxin D is a potent bioactive compound that is isolated from the marine cyanobacterium Lyngbya sp. and has demonstrated significant cytotoxicity against human lung cancer cells [27]. Four bioactive compounds, Dudawalamides A-D, isolated from Moorea producens were proven to be effective antiparasitic agents. All four of these compounds demonstrated potent activity, with IC50 ≥ 10 mM [28].
2. History and Existence
The organized structure and differentiation in the morphology of cyanobacteria, and their dominance in paleontological history [29][36], are due to the vital role that the unique cyanobacterial metabolism played in ancient ecosystems. This is also influenced by the selectivity of preservation and fossilization for particular environments or cyanobacterial taxa. The cyanobacterial fossil record is one of the oldest, reaching back to approximately 3500 Ma [30][37]. Fossil identification occurs by comparing old samples (established specimens existing in the herbarium) with modern forms in the natural population [30][31][37,38]. Scientists have devoted their efforts to documenting cyanobacteria across different eras (Figure 1). Several studies have documented that the most ancient in disputable fossil cyanobacteria were recorded in the Proterozoic Eon. Eoentophysalis belcherensis Hofmann was the first colonial coccoid microfossil present in this period. Their existence has been reported in intertidal and shallow subtidal environments on a carbonate platform in Canada [31][32][38,39]. Other fossils were recorded by Lanier (1989) as dating back to the Paleoproterozoic era. These fossils include vertically and horizontally oriented filaments, viz. Gunflintiaminuta, suggesting photoautotrophic cyanobacteria [33][40]. During the early Mesoproterozoic era, the cyanobacterial population, including entophysalidacean, oscillatoriacean, and nostocalean cyanobacteria, were widely spread across all possible ecological niches, from supratidal flats to open shelf marine environments [34][35][41,42]. The formation of distinct carbonate precipitates in this era helped fossilize a member of what was named the Archaeoellipsoides cyanobacterial genus in India [36][43]. Although they share many similarities with the extant Synechococcus genus, the samples show residues of akinetes resembling cyanobacteria Nostoc or Stigonema [29][37][36,44]. The late Mesoproterozoic era shows evidence of the existence of mat-dwelling and planktic chroococcacean cyanobacteria, with some cyanobacteria recognizable today dating back to the early Mesoproterozoic era. A distinctive feature of the evolution of cyanobacteria is the presence of a stalked cyanobacterium Polybessurus bipartitus [34][41]. The diversification of protists represents a critical event for developing the biosphere, and this has been documented as occurring in the era now named the Neoproterozoic era. Some new morphological varieties of cyanobacterial fossil debuted in this era, i.e., the remains of the stalked cyanobacterium Polybessurus Fairchild ex Green et al., which can sometimes be found in sediments [29][36]. In the near past, morphological criteria were solely used to classify cyanobacteria taxonomically; however, in the modern taxonomy, more adequate and complementary tools have been employed [38][45]. In the last few decades, the criteria of cyanobacterial taxonomic classification have radically changed after applying data obtained from electron microscopic studies along with phylogenetic analyses, mainly derived from molecular sequencing [39][46]. Modern techniques, such as the molecular method combined with traditional cyanobacterial morphological and ecological diversity studies, as well as the re-evaluation and redefinition of contemporary typical characters and markers of cyanobacterial entities in various biotopes, are inevitable taxonomic criteria for the primary classification. The molecular method used 16S rRNA gene sequencing as a standard, particularly at the genus level. The 16S rRNA gene sequencing clusters correspond to the traditional cyanobacterial taxa, which are identified by distinctive phenotypic features [5][6][5,6]. The combination of molecular methods with cyanobacterial taxonomy advanced important key aspects of cyanobacterial systematics as follows: Firstly, regarding various taxonomic units, the morphological characters should concur with their phylogenetic position. Secondly, phylogenetic relationships among cyanobacteria were discovered using combining molecular and phenotypic analyses, and a highly genetic variety was identified, particularly in coccoid and simple filamentous and pseudo-filamentous types [40][47]. Finally, a number of studies concluded that the fossil record of cyanobacteria, along with the modern methods of re-evaluation and redefinition of contemporary typical characters, helped scientists understand the complexity and diversity present in the history of evolution.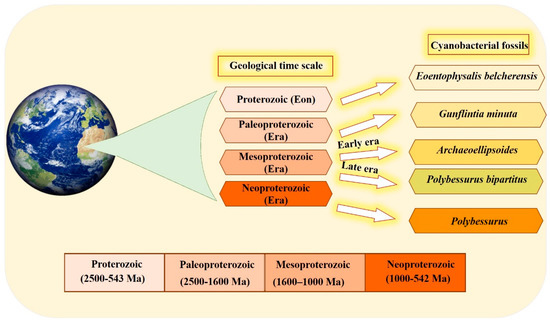
Figure 1. Timeline of the different cyanobacterial fossils recorded through different eras, mainly over the period ranging from 2500 to 542 million years ago, using a geological time scale.
3. Mechanism of Marine Cyanobacteria in Nitrogen Fixation
Cyanobacteria are recognized as the main N2-fixing microorganisms in the marine environment that participate in the universal nitrogen cycle [10][41][10,81]. N2-fixing cyanobacteria are grouped into filamentous non-heterocyst, unicellular cyanobacteria, and filamentous heterocyst-forming symbionts Figure 2 [10]. Regarding filamentous non-heterocyst, the genus Trichodesmium represents the most conspicuous marine organisms in terms of N2 fixing [42][82]. They are estimated to be responsible for approximately 50% of the global natural N2 fixation. Their nitrogen fixation process occurs during the day at temperatures ranging between 24–30°C, but stops at night due to inactivation and degradation of the nitrogenase enzyme [43][44][31,83]. Where N2 fixation occurs in differentiated cells of Trichodesmium spp., these cells consistently remain in clusters of about 3 to 20 [45][84]. The N products, such as ammonia (NH3), ammonium (NH4+), or the amino acid glutamine, are directly produced as a result of the reduction of N2 [46][47][85,86]. The following synthesis of glutamine and glutamate by a glutamine synthetase/glutamine oxoglutarate aminotransferase (GS/GOGAT) reaction also requires energy in the form of 1 ATP and 1 NADPH + H+. Thus, Trichodesmium’s capacity to fix nitrogen is highly reliant on the bioavailability of energy [48][87]. Unicellular N2-fixing cyanobacteria were first discovered through the amplification of nitrogenase genes and gene transcripts (mRNAs) from oceanic water samples [49][88]. According to recent studies, unicellular cyanobacteria (UCYN) have a high ability to fix N2 within a cell-size fraction below 10 μm. These unicellular cyanobacteria have been classified into three groups based on their nifH gene phylogeny: UCYN-A, UCYN-B, and UCYN-C [49][50][51][88,89,90]. In the UCYN-A group, cyanobacterial nitrogenase gene sequences were most tightly linked to sequences from the marine unicellular cyanobacterium Cyanothece sp. strain ATCC 51142 [52][91]. Despite numerous attempts, UCYN-A cyanobacteria have not been successfully cultivated [53][92]. UCYN-A cyanobacteria have genotypes that have not previously been identified in free-living cyanobacteria, and they lack the genetic ability for oxygenic photosynthesis. Furthermore, nitrogenase genes are most abundant in UCYN-A during the light period, and thus can fix N2 during daylight [51][90]. UCYN-B is a group of free-living unicellular cyanobacteria that fix N2 [54][93]. By studying the geographical populations of these free-living creatures, it may be concluded that they significantly contribute to the global N2 fixation process [55][94]. UCYN-B have only been identified in surface water and at 25% light depths (14 m) and is small in size (<10 μm) [56][57][95,96]. They fix N2 during the night [10]. In UCYN-B (Crocosphaera watsonii), nitrogenase enzyme synthesis begins just before nightfall to prepare for the upcoming N2 fixing activity during the night [58][97]. UCYN-C is a group of unicellular free-living cyanobacteria that fix N2 during the night [55][59][94,98]. It includes several cultivated cyanobacteria, such as Cyanothece sp. strain ATCC51142 and TW3 [60][61][99,100]. By 1993, Reddy and colleagues isolated a unicellular cyanobacterium, Cyanothece sp. ATCC51142, on the intertidal sands of the Texas Gulf Coast [62][101]. Their symbiotic associations play vital roles in chemical defense, as well as supplying partners with energy and organic products of carbon or nitrogen fixation [63][102]. In numerous oceanic diatom taxa, heterocyst-forming cyanobacterial symbionts are frequently observed [64][103]. In the microenvironment of a photosynthetic symbiotic partner cell, such as diatoms, heterocyst-forming cyanobacteria have a beneficial effect, where the oxygen-sensitive nitrogenase protein is inhibited as a result of O2 concentrations [10]. Richelia intracellularis J. Schmidt 1901 is a filamentous cyanobacteria frequently found either free-living or in symbiosis with the diatoms Rhizosolenia Brightwell 1858 and Chaetoceros Ehrenberg 1844 in the plankton of the warm oceans [63][102]. Cyanobacteria nitrogen fixation has various impacts that can be explained as follows: firstly, cyanobacteria are significant bioavailable nitrogen suppliers to the pelagic and benthic food webs that support fish productivity by fixing dissolved N2. The food web benefits from bioavailable nitrogen in addition to the fresh or decaying filamentous cyanobacteria to nourish various invertebrates and release more nitrogen by cyanobacterial cells. Secondly, their ability to get beyond summertime nitrogen limitation by the fixation of dissolved nitrogen. Thirdly, they can contribute to enhancing productivity in different agricultural and ecological situations by building up soil fertility and increasing yield [65][66][67][104,105,106]. ThWe researchers conclude that only filamentous non-heterocyst cases have contributed to the explanation of the mechanism of nitrogen fixation. On the other hand, there are limitations in the data related to unicellular cyanobacteria, and filamentous heterocyst-forming symbionts, as there are no papers explaining the role of the nitrogen fixation mechanism in these cases. Taken together, nitrogen fixation has important impacts in various fields, i.e., agriculture and ecology, reflecting a potential benefit on the economy.
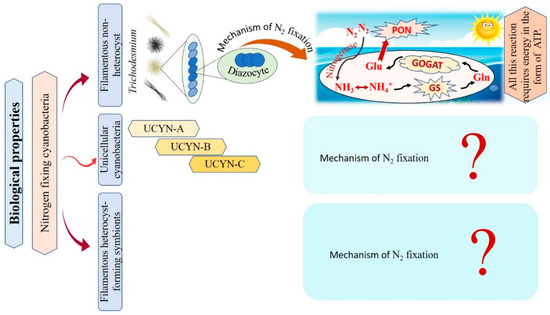

Figure 2.
Flowchart for the different types of nitrogen-fixative cyanobacteria with their mechanisms of action.
4. Mechanism of Marine Cyanobacteria in CO2 Fixation
Industrialization and the burning of fossil fuels are to blame for the alarmingly high CO2 levels in the atmosphere. CO2 concentrations in surface waters have increased with the rise in atmospheric CO2 during the past century. Cyanobacteria are one of the most promising organisms for CO2 capture [68][69][70][107,108,109]. The most common photoautotrophic lineage on Earth comprises cyanobacteria. Their effectiveness in photoautotrophism relies on a collection of adaptations known as the CO2-concentrating mechanism (CCM). The CCM aims to increase the efficiency of CO2 fixation by promoting the carboxylase reaction through improving the chemical conditions around the main CO2-fixing enzyme, D-ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO), and suppressing the oxygenase reaction [71][110]. In cyanobacteria, RubisCO is enclosed within a class of protein-rich structures known as carboxysomes. The selectively permeable protein shell of the carboxysomes contains the CO2-fixation enzyme, as well as the carbonic anhydrase enzyme, which provides CO2 from a cytoplasmic bicarbonate pool [71][72][110,111]. The carboxysomes have two main types, α-cyanobacteria, which are found in marine water and β-cyanobacteria, present in fresh water [72][111]. In α-cyanobacteria, the RubisCO is categorized as (RuBisCO Form IA) [73][112]. In α-cyanobacteria, up to two different types of plasma membrane-associated bicarbonate transporters, SbtA2 (high-affinity Na+/HCO3−) and BicA2 (medium to low-affinity Na+/HCO3−) promote CO2 fixation [71][110]. In the first stage of the (CCM), bicarbonate is concentrated inside the cell via transporters in the cell membrane. In the second stage of (CCM), the carboxysome plays a vital role in the enhancement of CO2 fixation by co-localizing the two enzymes (CA) and (RuBisCO). Bicarbonate is assumed to enter the carboxysome through proteinaceous shell pores, and once inside, it is converted to CO2 and used by RuBisCO. The conversion of HCO3− to CO2 is catalyzed by the CA enzyme. After CO2 fixation, some of it exits into the cytosol, while some promptly combines with RuBP to form phosphoglycolate Figure 3 [72][74][111,113]. The components of the cyanobacterial CO2-concentrating mechanism (CCM) have a vital role in the improvement of the efficiency of photosynthetic CO2 fixation in the chloroplasts of crop plants via specific cyanobacterial transporters, accordingly leading to better crop yields [75][114]. Finally, thwe researchers cconclude that the importance of CO2 revolves around the conversion of inorganic carbon to organic carbon through vital processes.
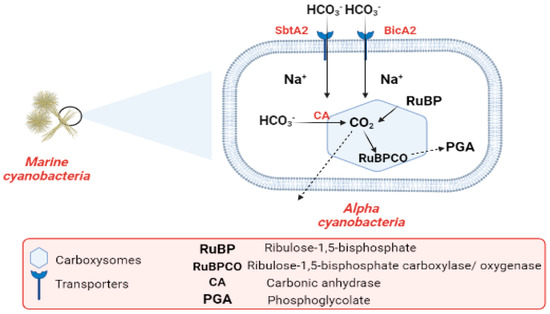

Figure 3.
Flow chart explaining the carbon cycle in marine cyanobacteria.
