Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Payal Ganguly and Version 2 by Jason Zhu.
Bone regeneration and repair are complex processes with the potential of added complications, like delayed repair, fracture non-union, and post-surgical infections. These conditions remain a challenge globally, pressurizing the economy and patients suffering from these conditions. Applications of nanotechnology (NBT) in the field of medicine have provided a medium for several approaches to support these global challenges. Tissue engineering is one such field that has been on the rise in the plastt three decades through the utilization of NBT for addressing the challenges related to bone regeneration.
- bone repair
- tissue engineering
- bone regeneration
- nanomedicine
- nanobiotechnology (NBT)
- nanoparticle (NP)
- regenerative medicine
- bone tissue engineering (BTE)
1. Introduction
Nanoscale materials have emerged as pivotal players in BTE due to their inherent advantages, including biocompatibility, biodegradability, mechanical tunability, and their capacity to mimic the natural bone ECM [1][2][3][4][5][28,37,38,39,40]. Several types of materials are being used either individually or more commonly in combination with materials providing complementary benefits towards the final scaffold. For example, several polymers, including natural polymers such as chitosan, gelatin, and collagen, are used in hydrogel preparation for BTE due to their biochemical and biophysical properties [6][41]. Other polymers, discussed below, were also found to be beneficial at nanoscale for applications in drug delivery and tissue engineering [7][42]. They may also be used in combination with harder materials, like bioactive glass, or metals, like titanium, zinc, strontium, and others in their nanoscale forms, offering biocompatibility, enhanced mechanical strength, and biodegradability towards scaffold formulation [8][9][43,44]. The various materials used for BTE, as shown in Figure 1, broadly explores polymeric and non-polymeric materials that have been used in BTE applications, specifically for scaffold formation.
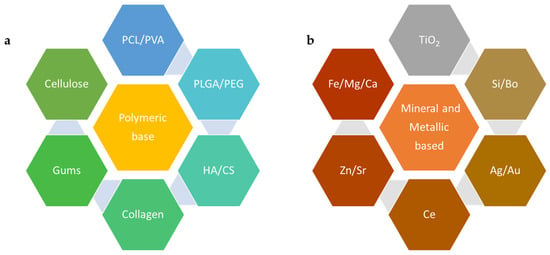
Figure 1. Example of materials used in nanoscale forms for BTE: (a)—polymeric materials and (b)—metallic elements, PCL—poly(ε-caprolactone), PVA—poly(vinyl alcohol), PLGA—poly(lactic-co-glycolic acid), PEG—polyethylene glycol, HA—hyaluronic acid, CS—chitosan, TiO2—titanium dioxide, Si—silicon, Bo—boron, Ag—argentum (silver), Au—aurum (gold), Ce—cerium, Zn—zinc, Sr—strontium, Fe—ferrous/ferric (Iron), Mg—magnesium, Ca—calcium.
2. Polymeric Materials Used at the Nanoscale for BTE
2.1. Synthetic Polymers
Polycaprolactone (ε-PCL) is a biodegradable, biocompatible polyester that has attracted substantial attention in BTE due to its adaptable biodegradation and ease of manipulation into NFs, NPs, and scaffolds [10][48]. A PCL fiber mesh fabricated via electrospinning demonstrated enhanced osteoblast cell proliferation and differentiation, whereby the structure mimicked the natural ECM, facilitating cell adhesion and growth essential for successful BTE [11][49]. Poly(lactic-co-glycolic acid) (PLGA), a copolymer of lactic and glycolic acids, is another example of a biodegradable and biocompatible polymer commonly used in BTE. Nanostructured PLGAs can be formed into NPs for drug delivery, NFs, or scaffolds for cell growth and have each contributed unique benefits to BTE [12][13][50,51].
PLGA NFs combined with nano hydroxyapatite (HA), a major inorganic component of bone, exhibited improved cell attachment, proliferation, and differentiation [14][52]. Polyethylene glycol (PEG) is another polymer that is often utilized in nanoscale form due to its excellent biocompatibility and resistance to protein adsorption. PEGylated gelatin nanospheres have been used to deliver BMP-2, which enhances bone regeneration in vivo, signifying the importance of PEG in nanoscale drug delivery systems for BTE [15][53]. Other nanoscale polymers, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly(vinyl alcohol) (PVA), and polyurethane (PU), are also being explored for their potential in BTE and have been discussed in great detail elsewhere [2][16][17][37,54,55]. When crafted into nanoscale forms, such as fibers or particles, these polymers show promising potential in promoting cell attachment, proliferation, differentiation, and delivering therapeutic agents, thereby boosting bone regeneration [8][9][18][19][20][43,44,56,57,58].
2.2. Natural Polymers
Chitosan (CS) is another example of a biopolymer exhibiting a multitude of favorable properties, including non-toxicity, biodegradability, biocompatibility [21][22][59,60], antifungal, antibacterial [23][24][61,62], and wound healing abilities. Therefore, CS is widely utilized in the biomedical, biotechnology, and pharmaceutical fields [25][26][63,64]. CS is a prime candidate for crafting potential bone scaffolds, particularly when paired with osteoconductive substances, such as HA [21][27][59,65] and poly(acrylic acid) (PAA) [28][66]. Studies have shown that CS-based bone scaffolds foster cell adhesion and osteoblast cell proliferation, creating mineralized bone matrices in vitro [29][67]. An enhancement in osteoblast cell proliferation was observed for CS composites that incorporated nanoHA, ultimately triggering bone regeneration within eight weeks as validated by micro-computed tomography [30][68]. The mechanical characteristics of CS scaffolds can be enhanced by crosslinking with substances possessing a minimum of two reactive functional groups, such as calcium phosphates, composites (nanozirconia and nanocalcium zirconate), and bioglass, leading to a superior performance compared to constructs consisting of only CS [31][69].
Cellulose is another widespread linear biopolymer that contributes significant tensile strength to trees and is present in various organisms, including marine species, bacteria, fungi, and even amoebas [32][70]. It is usually characterized by long fibrils that comprise various crystalline and amorphous regions. By subjecting the cellulose pulp to mechanical or chemical alterations, it is feasible to extract tiny crystalline cellulose components. These diminutive cellulose polymers, referred to as cellulose nanocrystals (CNCs), typically exhibit diameters of 2–20 nm and a length distribution of 100–600 nm [33][71]. The functionalization of CNCs with sulphate or phosphate ester groups can be achieved through sulfuric or phosphoric acid hydrolyses [34][72]. These modified scaffolds reveal superior crosslinking and, subsequently, enhanced mechanical properties compared to those that are unmodified.
Furthermore, compared with phosphate ester-modified aerogels, aerogels crafted from sulphate ester-treated CNCs display greater compressive strength, porosity, and crosslinking [35][73]. These sulphate ester-modified CNCs (S–CNCs) possess in vivo osteoconductive properties, enabling bone growth at the defect location. After three weeks, S–CNC aerogels can create a bone volume percentage that exceeds controls by 33% and can see a size increase of 50% after twelve weeks. Comprehensive research into cell–interface interactions and in vivo scaffold degradation is crucial. Scaffolds constructed using modified CNCs with bone tissue-enhancing properties are emerging as promising solutions for BTE applications [36][47]. Various methods, like electrospinning or 3D printing, may be applied to fabricate polymeric scaffolds and the recent advances in these applications have been outlined elsewhere [37][38][74,75].
3. Mineral-Based and Metallic Nanoscale Materials Used for BTE
NPs obtained from minerals, such as zinc, silica, or calcium phosphate particles, are classified as nanoscale materials where their primary role is to provide vital ions for tissue generation. They do so by enhancing the mechanical properties of 3D-printed scaffolds. Mineral-derived nanomaterials can be produced by several techniques, such as plasma spraying, milling, and precipitation from solution [39][76]. Similarly, metallic nanoscale formulations of gold (Au), silver (Ag), and titanium (Ti) have been explored at length for their enhanced functionalities.
3.1. Mineral Nanoparticles
Calcium Phosphate
Adding mineral-based nanomaterials to both the surface and bulk of 3D-printed scaffolds has been shown to improve the overall functionality of the scaffolds. Nanomaterials derived from calcium phosphate (CaP) are often added to the 3D-printed scaffolds since bone is inherently composed of CaP crystals (70%) and 30% of organic collagen fibrils [40][77]. In 2017, Chen et al. generated a biomimetic composite scaffold of collagen and biphasic calcium phosphate nanoparticles (BCP NPs). They engineered these scaffolds to release dexamethasone (DEX) during preparation and hybridized this with collagen scaffolds. The subcutaneous implantation of the composite scaffolds at the dorsal side of athymic nude mice demonstrated the regeneration of the ectopic bone tissue. When used for the 3D culture of human BM-derived MSCs, these scaffolds demonstrated enhanced biocompatibility and promoted the osteogenic differentiation of hMSCs [41][78]. Another study by Sokolova et al. in 2020 used scaffolds of PLGA and nanohydroxyapatite (nHAP) (85:15) combined with DNA-loaded CaP NPs. The results suggested increased cytocompatibility and rate of gene transfection into cells indicating their favorable application as a scaffold for BTE or a bone substitution material [42][79].
Silicon Dioxide (SiO2)
Silica NPs are an example of inorganic NPs. Silica particles have been shown to promote osteoblast differentiation while inhibiting osteoclast differentiation [43][80]. A recent study by Echazú et al. in 2022 [44][81] combined soluble silica particles and a CS polymer to engineer a potential bone substitute. This was implanted into the medullary compartment of both tibiae in Wistar rats and investigated for cytotoxicity and biocompatibility. The results indicated successful new bone formation at the tissue–biocomposite interface (osseointegration) [44][81]. Another study conducted in 2023 by Shuai et al. used poly(L-lactic acid) (PLLA)-based bone scaffolds and combined SiO2 NPs with graphene oxide (GO) NS to promote the dispersion of GO on the biopolymer bone scaffold. The optimization of the adequate dispersion of GO with SiO2 enhanced the mechanical properties and cytocompatibility of the scaffold, making it a potential candidate for BTE applications [45][82].
Zinc Oxide (ZnO)
Zinc is one of the minerals present in bone in the form of trace elements. It promotes bone density and the prevention of bone loss [46][83]. It also activates proteins involved in bone homeostasis as a part of inorganic minerals. ZnO NPs exhibit low toxicity, act as antibacterial agents, and exhibit optimal biological compatibility and chemical stability. They stimulate osteogenesis and hence have the potential to accelerate bone growth and mineralization [47][48][84,85]. In 2020, Cho et al. investigated the cell proliferation and antibacterial activities of a PCL/nanoHA scaffold doped with ZnO. Their results indicated that the scaffolds with 100 nm-thick ZnO coatings showed enhanced antibacterial cell proliferation activities and mechanical properties [49][86]. In 2017, Forero et al. studied the effect of CS/gelatin/nanoHA scaffolds containing a nano-copper–zinc alloy for BTE. These scaffolds increased the proliferation and adhesion of mouse embryonic fibroblasts and induced osteogenic differentiation. After an in vivo subcutaneous implant, they induced the growth of surrounding tissues and promoted granulation tissue formation [50][87]. The use of ZnO NPs in the field of BTE is very limited, but promising, with novel advancements being made using hybrid mix of compatible biopolymers.
3.2. Metallic NPs
A high penetrating ability and surface area coupled with improved cell adhesion, differentiation, and growth suggests that metal NPs are one of the ideal candidates for scaffold fabrication in BTE. Ag and Au are the most common osteogenic agents used in this category. They exhibit a high surface area, enhanced antibacterial properties, biocompatibility, and surface reactive features in order to identify pathogenic viruses [51][88] and intracellular targets [52][89].
Silver (Ag)
Silver (metal or salt) has been used as an antibacterial agent in implants [53][54][90,91]. Ag ions can penetrate the bacterial cell wall and cause growth inhibition. Ag-based scaffolds are highly potent with enhanced cell adhesion and cytocompatibility, improved osteogenesis and osteo-conductivity, acceptable mechanical strength, an ability to bridge oxygen molecules, and low toxicity [55][56][57][92,93,94]. Akturk et al. optimized the protocol to generate an antibacterial gelatin nanocomposite membrane for BTE applications. They used soluble starch-coated Ag NPs and bioactive glass particles incorporated into gelatin to fabricate a nanohybrid nanocomposite fiber [58][95]. Another study investigated the use of CS-silver polymeric scaffolds in BTE. These scaffolds demonstrated enhanced matrix mineralization and osteogenic differentiation (marked by the upregulation of marker genes Runt-related transcription factor 2 (Runx2), type-1 collagen (Col-I), alkaline phosphatase (ALP) activity, and secreted osteocalcin (OCN)) [59][96].
Gold (Au)
Gold NPs exhibit low toxicity, high antibacterial effects, and biocompatibility in the scaffold environment [60][97]. While some studies indicate dose-dependent cytotoxicity in human cell lines marked by membrane damage and reactive oxidative species (ROS) generation [61][62][98,99], this property of Au NPs has often been useful to destroy cancer cells [63][100]. Thus, ensuring the appropriate size and dosing of Au NP plays a crucial role in BTE applications. Nekounam et al. demonstrated an optimized protocol for the fabrication of a carbon NF/Au NP-based scaffold that supported cell proliferation and indicated low toxicity in bone cells [64][101]. Au NPs that are smaller in size (<10 nm) have been used for miRNA delivery to the nucleus. Yu et al. in 2017 created nanohybrids using polyethylenimine, liposomes coated on the surface of Au NPs. After the release from endosomes inside the cells, miR-5106 was able to facilitate BM stem cell osteoblastic differentiation, which further aided in mineralization [65][102].
Titanium Dioxide (TiO2)
TiO2 NPs are bioinert cell carrier materials with improved permeability and high biocompatibility. They are available in the form of nanocrystals and exhibit antibacterial and antiseptic properties making them a promising scaffolding material for bone tissue repair [66][103]. A study published in 2018 described the methodology to fabricate a lightweight and economical TiO2/CS scaffold by freeze-drying [67][104]. An in vitro study conducted by Pattanashetti et al. in 2020 determined the ideal weight percentages of TiO2 NPs that needed to be crosslinked in a PVA matrix. These nanofibrous scaffolds were developed using electrospinning and the results indicated that 0.1 g of TiO2 exhibited enhanced mechanical stability and a subsequent slow rate of degradation in the scaffolds. These scaffolds were found to be viable and non-toxic to the cells, as observed by the MTT assay using MG-63 osteosarcoma cell lines [68][105].
