Over the past century, our genetic background has not changed, but chronic diseases are on the rise globally. In addition to the genetic component, the critical factors for many diseases are lifestyle, eating changes, exposure to drugs, xenobiotics, alcohol, smoking, polluted air, etc. These techniques include genome-wide association studies (GWAS) that allow the identification of unknown genetic risk factors, positional cloning of unknown genes associated with different diseases, the gene tests for single nucleotide variants (SNVs), and next-generation sequencing (NGS) of selected genes or/and the entire genome. Gut microbiome composition and its metabolites are not only regulating factors in carcinogenesis (including de novo after liver transplantation) but also in xenobiotics and anticancer treatment failure, observations that may be related to the genetic background of the individuals.
1. GWAS Loci for Predisposition and Susceptibility of NAFLD
NAFLD is the most common form of metabolic disease worldwide, occurring in 17–30% of the population
[1][2][3][91,92,93]. The etiology is thought to be multifactorial, and the heritability estimates typically range from 20 to 70%, depending on study design, methods, age, and ethnicity
[4][5][94,95]. GWASs of NAFLD are relatively small because of the lack of abdominal MRI and/or liver biopsy data
[6][7][8][9][10][11][12][13][14][15][90,96,97,98,99,100,101,102,103,104].
Several recent larger GWAS studies have been conducted that have identified multiple different risk loci for NAFLD
[16][17][18][105,106,107]. Of all investigated genetic variants, patatin-like phospholipase domain-containing protein 3 (
PNPLA3) appears to be a major common determinant of NAFLD. In 2008, the first GWAS identified a significant association between rs738409 encoding Ile148Met (I148M) and NAFLD, independent of alcohol use, body mass index, and diabetes
[6][90]. Subsequently, multiple GWAS studies established that
PNPLA3 I148M is strongly associated with the entire spectrum of NAFLD and genetic predisposition to disease and HCC
[4][19][94,108].
Several studies have identified the
MBOAT7 variant rs641738 as a risk locus for NAFLD development and disease severity
[20][21][109,110], although this variant has previously been associated with alcohol-induced cirrhosis
[22][111].
A multi-ancestry GWAS conducted in the Million Veterans Program included 90,408 cases of chronic alanine aminotransferase elevation and 128,187 controls
[23][112]. Seventy-seven significant genome-wide loci were identified, 25 without previous NAFLD or alanine aminotransferase associations. In two additional external NAFLD cohorts, 17 SNPs were replicated, 9 of which were novel. A pleiotropic analysis showed 61 multi-ancestry and the 17 SNPs were associated with metabolic or/and inflammatory phenotypes. Miao et al. used the UK Biobank (UKB) to estimate the NAFLD status based on anthropometric measures and serum characteristics
[16][105]. They identified 94 NAFLD loci. Most were not previously identified but related to coronary artery disease (CAD)
[16][105].
In 2019, Nimjou et al. published a report from a GWAS using adult and pediatric participants from the eMERGE network (Electronic Medical Records and Genomics Network)
[18][107]. The study confirmed the association for the
PNPLA3 gene in adult and pediatric patients, like disease severity locus. A conducted GWAS on NAFLD cases and healthy controls from the UK Biobank also identified genetic risk variants
[18][107]. Over 9 million variants were estimated by logistic regression adjusted for sex, age, genetic components, and genotyping batch. A meta-analysis also identified six risk loci (
APOE,
PNPLA3,
TM6SF2,
GCKR,
MARC1, and
TRIB1). All these six susceptibility loci were significant. This GWAS also confirmed that the
ϵ4 allele of
APOE is associated with protection against NAFLD
[18][107].
It also has been established that different cytokines are involved in the pathogenesis of NAFLD
[24][113]. Of all, IL-6 was significantly increased in the liver of NAFLD individuals and correlated with disease severity
[25][114]. More recently, a human pluripotent stem cell model of NAFLD was developed that is suitable for the mechanical dissection of genetic variants
[26][115]. The study shows that the strong association between rs738409 C > G in
PNPLA3 and susceptibility to NAFLD is caused by increased IL-6/STAT3 activity, which leads to accelerated disease progression. The same study found that global blocking of IL-6 signaling reduced NAFLD development and progression. Because the IL-6 signaling pathway and the
PNPLA3I148M variant are associated with developing HCC, this model can be used to study variants for other liver diseases than NAFLD.
2. NAFLD GWAS Loci Overlap with GWAS Loci for Liver Enzymes, ALD and HCC
Some NAFLD GWAS loci overlap with GWAS loci liver enzymes—ALT, AST, GGT, and alkaline phosphatase (ALP). They are the most commonly used laboratory markers of liver disease, and the variations in their levels are heritable
[27][28][29][116,117,118]. Combined GWAS of AST and ALT have revealed genetic associations with the
PNPLA3 gene
[6][90] and
HSD17B13 [30][119]. A recent study conducted a GWAS meta-analysis on serum ALT and AST activities in 411,048 subjects. They identified 100 loci associated with liver enzymes. The strongest association observed was with a rare missense variant in
SLC30A10 [31][120].
Another recent study on liver enzymes identified 172
ALT, 199
AST, and 216
ALP loci
[32][121]. Of the mentioned loci, 160
ALT, 190
AST, and 199
ALP loci are novel, and 153 variants are significant. Data on the specific genetic risk variants involved in ALD pathogenesis exist. Different GWASs showed that the rs738409 variant in
PNPLA3 was associated with ALD and alcohol-related cirrhosis
[33][34][35][124,125,126]. The genes
TM6SF2 and
MBOAT7 are also the genetic modifiers of ALD
[22][111]. The MBOAT7 (rs641738) showed about 80% increased risk of HCC in NAFLD patients and the development of HCC in ALD patients
[36][127]. The different combinations of the three variants may increase the risk of progressive liver alteration and HCC
[37][38][39][40][128,129,130,131].
Stickel et al. found that the development of HCC was associated with the same genetic variants—
PNPLA3 (rs738409) and
TM6SF2 (rs58542926)
[41][132]. Patients with alcohol-related cirrhosis who carry these variants also have an increased risk of HCC
[40][131].
PNPLA3,
TM6SF2, and
MBOAT7 appear to be genetic modifiers of both ALD and NAFLD and share some biological pathways and histological patterns
[42][43][133,134].
3. GWAS Loci for Predisposition and Susceptibility of ALD
Other genetic variants involved in ALD have also been described. The studies had significantly small sample sizes, and some results were not replicated. For example, a non-synonymous variant (rs4880) in the
SOD2 gene has been associated with progressive ALD, but the data have not been confirmed
[44][45][135,136].
Risk alleles in the
IL10,
TNFα,
TGFβ, and
MMP-3 genes have been investigated for association with alcohol-related liver injury
[46][47][48][49][50][137,138,139,140,141].
The data from these studies are not very conclusive and require further research. In addition, the rs2228603 in the
NCAN gene is associated with NAFLD but was found to be a risk factor for HCC, even in patients affected by alcohol-related cirrhosis
[51][52][142,143].
Another recent study found three loci,
ZNF827,
GGT1, and
HNF1A, to be significantly associated with ALD risk
[53][144]. Other GWASs for ALD revealed candidate genes such as
GABRB1,
DRD4 and
TH,
PECR,
PDLIM5,
METAP,
ADH1C, etc.
[54][55][56][145,146,147].
4. GWAS Loci for Predisposition and Susceptibility of ALC
Unlike other liver diseases, very few genetic variants that influence the risk of cirrhosis have been identified. A GWAS for alcohol-related cirrhosis (ALC) in European descent identified the
MBOAT7/TMC4 locus as a new genetic risk factor
[22][111].
Another GWAS/meta-analysis conducted in 2021 by Schwantes-An et al. analyzed ALC patients and healthy subjects who drank heavily
[57][148]. A significant risk association was found again with
PNPLA3 and
HSD17B13, and a protective association for
FAF2. Meta-analysis confirmed GWAS significance for these three loci. Two other known loci,
SERPINA1 and
SUGP1/TM6SF2, were also GWAS significant in this meta-analysis.
Emdin et al. identified 12 independent genetic variants associated with cirrhosis risk—5 previously reported and 7 newly discovered
[58][149]. Recently identified variants include the missense variant in
APOE (Cys130Arg) and a non-coding variant located in the 3′ untranslated region of the
EFNA1 gene (rs12904)
[58][149].
A conducted GWAS analysis identified five previously associated variants in the
MARC1 (p.Ala165Thr),
PNPLA3 (p.Ile148Met),
TM6SF2 (p.Glu167Lys),
HSD17B13 (rs6834314), and
SERPINA1 (p.Gly366Lys) gene regions
[30][59][119,150]. The previously reported variant in
MBOAT7 (rs641738) was also associated with cirrhosis. However, it did not reach genome-wide significance
[14][103]. A recent report identified a new variant near the
HNRNPUL1 (rs15052) associated with alcoholic cirrhosis
[60][151].
5. GWAS Loci with Significant Association with HCC
Five SNPs were found, three in
PNPLA3 and two in
SAMM50, with significant association with NCC in conducted GWAS
[61][152]. The SNPs in
PNPLA3 are rs2281135, rs2896019, and rs4823173. The two SNPs in
SAMM50 are rs3761472 and rs3827385. They were replicated in a cohort study in Singapore and a US case-control study, indicating that these SNPs were significantly associated with HCC. Other GWAS studies identified
WNT3A-WNT9A (rs708113)
[62][153]. They supported the previously reported regions associated with alcohol-related hepatocellular carcinoma risk—
TM6SF2 (rs58542926) and
PNPLA3 (rs738409)
[37][63][128,154]. These two missense variations are well studied and shown to contribute to chronic liver damage by accumulating fat. Their role in liver carcinogenesis is still under investigation and remains unclear
[64][155]. The three variants reached GWAS significance in the meta-analysis. A recent study also revealed these variants for alcohol-related HCC and described several previously reported variants
[65][156].
As well as excessive alcohol consumption, other risk factors for HCC are chronic hepatitis B and C virus infections, obesity, aflatoxin exposure, metabolic diseases, and individual genetic predisposition. Various GWASs have been conducted for these factors, and genetic loci and their association with HCC have been well established
[66][157]. More studies are needed for a more comprehensive understanding of the genetic mechanisms underlying alcohol-related HCC, leading to better prevention and early diagnosis.
6. Other Genetic Loci Related to Different Forms of Liver Diseases
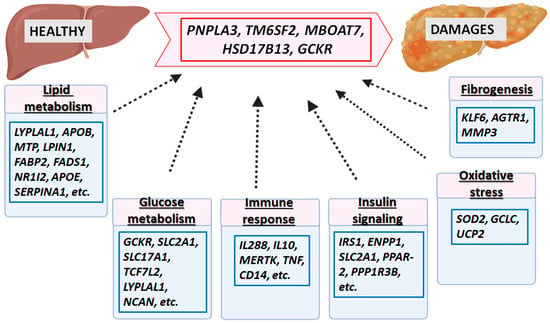
Genetic factors related to the progression of different forms of liver diseases (ALD, NAFLD, cirrhosis, HCC, etc.) interact with genes involved in glucose and lipid metabolism, insulin signal pathways, oxidative stress, fibrogenesis, immune response, and inflammation. The liver, metabolic, and inflammatory traits are shown in
Figure 14. The most significant genes associated with increased liver fat, cirrhosis, HCC, etc., are
PNPLA3,
TM6SF2,
HSD17B13,
GCKR, and
MBOAT7. Others, such as
MARC1,
SERPINA1,
APOE,
ALDH1B,
GPAM,
HNF1A, etc., are less stable. Rare variants such as MTTP and APOB are associated with an increased risk of liver fat damage and HCC. In addition, other genes related to the progression of NAFLD and involved in regulating lipid metabolism are
LYPLAL1,
APOB,
MTP,
LPIN1, and
UCP2.
GCKR has been reported to regulate glucose metabolism and lipogenesis,
IL28B and
MERTK in innate immunity,
SOD2 in oxidative stress,
ENPP1 and
IRS1 in insulin signaling, and
KLF6 in fibrogenesis. They are also associated with the progression of NAFLD
[67][68][69][158,159,160].
Figure 14. Major genetic factors involved in the pathogenesis of liver disease. The most significant genes associated with the pathology of liver diseases are given in the red box. In blue boxes are given genes involved in glucose and lipid metabolism, insulin signaling pathway, oxidative stress, fibrogenesis, immune response, and inflammation, which interact with the most important genetic factors associated with the progression of different forms of liver diseases (ALD, NAFLD, cirrhosis, HCC, etc.).
These genes also influenced microbiome composition in host genome–microbiome association studies
[70][161]. In genetically susceptible individuals, environmental triggers may create the inability to distinguish between commensal and pathogenic microbiome components, which may cause immunological diseases. Host genetics are the leading cause of pathogenesis. The gut microbiome’s role in both scenarios raises an important question: can it be used as a diagnostic biomarker or therapeutic target for autoimmune liver diseases? Except for genome editing, a person’s genome is static. Probiotics, antibiotics, diet, immunization, and transplantation can alter or reassemble the human super-organism’s “other genome”, the microbiome.