Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Manikant Tripathi and Version 2 by Peter Tang.
Different types of dyes emanating from textile wastewater have adverse effects on the aquatic environment. Various methods including physical, chemical, and biological strategies are applied in order to reduce the amount of dye pollution in the environment. The development of economical, ecologically acceptable, and efficient strategies for treating dye-containing wastewater is necessary. It has been shown that microbial communities have significant potential for the remediation of hazardous dyes in an environmentally friendly manner.
- bioremediation
- dye degradation
- environmental pollutants
- genetic engineering
- microbial fuel cells
- nanotechnology
- textile wastewater treatment
1. Introduction
Industrial wastewater is very harmful for our environment and causes various adverse impacts on the ecosystem. Most wastewater is generated from the textile, cosmetics, printing, paper, and rubber industries [1][2]. The textile industry generates huge amounts of highly toxic chemicals that are released at various stages of processing [2][5]. In addition, it is well documented that textile dyes, without proper treatment, pose serious eco-toxicological threats to living forms [3][4][6,7].
Textile dye-containing wastewater is also one of the major sources of water pollution, and it affects the environment by disturbing aquatic life, inhibiting the photosynthesis process in aquatic plants, and affects human health by causing breathing difficulties, irregular heartbeats, skin rashes, dizziness, and cancer [3][5][6][4,6,8]. Different types of dyes are used in the textile industry. Most of the common dyes applied in the textile industry are called azo dyes [7][8][9,10].
Since ancient times, mankind has known about and utilized dyes [9][11]. In many manufacturing sectors, including the paper industry and many more, a number of techniques are effective in removing dyes [10][11][12][12,13,14]. It has been found that the dyes used in the textile industry have high toxicity and a potential carcinogenic nature [6][13][8,15]. The textile industry is responsible for a broad range of impacts on the environment [14][16]. Textile dyes can cause diseases ranging from dermatitis to central nervous system disorders [15][17]. Consequently, treating toxic wastewater containing different types of textile dyes is a very compelling issue currently [16][17][18][18,19,20]. Accommodating a single or multiple azo groups, azo dyes constitute 60–70% of known dye structures [19][20][21][21,22,23].
The treatment of dye-containing wastewater occurs through various methods like physical, chemical, biological, and recent advanced technologies such as using nanotechnology, microbial biosorbents, microbial films, genetic engineering, plant–microbe-mediated techniques, and others [5][12][4,14]. In recent studies, researchers have reported the use of chemical-based methods for the remediation of pollutants from wastewater [22][23][24][24,25,26]. Metal-based organic frameworks are also used as sensitive and selective sensors for detecting hazardous pollutants [25][27]. However, physical and chemical methods are not cost-effective [3][6].
Microbial processes using algae, bacteria, yeast, and fungi are low-cost and can be successfully utilized for dye remediation. Microorganisms are capable of breaking down the azo bonds present in dye molecules through their enzymatic activity [3][26][6,29]. Advanced treatment strategies using microorganisms can make the remediation process more effective, while appropriate environmental conditions should also be ensured for effective bioremediation [27][30].
Pollutants such as dyes from the textile industry are present in wastewater. Various techniques, such as physical, chemical, and biological methods, are used for the decontamination of dyes in aquatic environments. The physical and chemical procedures used to treat textile wastewater are inadequate and not always environmentally feasible. A safe method to detoxify dyes from wastewater is bioremediation technology using microorganisms or plants. Nowadays, advanced technologies such as bioremediation, including nanotechnology, bioreactors, microbial fuel cells, genetic engineering, and others, are being applied in the treatment of dye pollution in the environment (Figure 12).
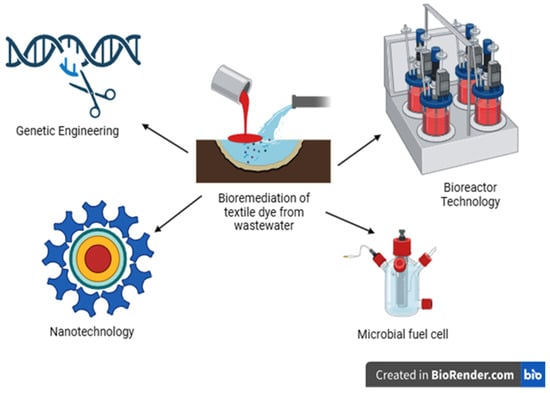

Figure 12.
Recent possible strategies for dyes remediation from aquatic environment.
2. Physical and Chemical Approaches
Adsorption, ion exchange, and membrane filtration are a few of the physical methods used in the remediation of environmental pollutants.
2.1. Adsorption
Adsorption is used for the management of industrial wastewater. Adsorption is a surface-based phenomenon in which the solid surface of the adsorbent attracts charged ions or molecules, which then adsorb onto the surface. Several types of forces are responsible for dye molecule adsorption, including hydrophobic, electrostatic interaction, hydrogen bonding, and van der Waals forces [28][37]. The adsorption process removes dyes from contaminated wastewater, and there is a possibility of upcycling the adsorbent for reuse in treatment [29][3]. The phenomenon of adsorption is dependent on the adsorbent, which contains pore-like structures that are required for the quick and systematic adsorption of dye molecules from wastewater [30][31][38,39]. Most adsorbents, such as silica gel, alumina, zeolite, and activated carbon, are commonly used for the removal of toxic dyes from contaminated wastewater [32][40]. Other mechanisms like complexation and ion exchange are applied in the remediation of dyes [33][34][35][41,42,43]. In their study, Briao et al. [36][44] found that ZSM-5 zeolite adsorbent is used in the treatment of dye-containing wastewater, such as basic fuchsin, crystal violet, and methylene blue, with a degradation percentage of 81.2%, 75.3%, and 86.6%, respectively. In another study, Madan et al. [37][45] reported 90% Congo red decolorization by ZnO, used as an adsorbent, while Harza et al. [38][46] reported Congo red dye remediation with the help of fly ash generated from a local powerplant. Several researchers have also reported different types of organic adsorbents and their composites for dye remediation from aqueous solutions, and some of the most interesting adsorbents are conducting polymers in their different forms, including powders and aerogels, as well as biopolymers [39][40][41][47,48,49].
2.2. Ion-Exchange Method
In the ion-exchange method, effective separation is accomplished by creating a complex bond between resin, which is a bed reactor, and a solute. Akpomie and Conardie [29][3] and Ahmad et al. [42][50] found that the process of dye removal in ion exchange mainly depends on strong interactions between charged molecules in the dye and the functional group of the resin. The cation exchangers and anion exchangers are used as resins to separate solutes with different surface charges [43][51]. Many researchers reported that dyes like acid orange 10 are removed by an anion-exchanger resin named Amberlite IRA-400, and the percentage of dye removed was 96.8% [44][52]. Another dye, acid black, was remediated at a rate of 100% by an anion exchanger that was synthesized using cellulose [45][53]. Disperse violet 28 dye is generally removed by cation-exchanger resin, and the dye removal percentage is 91.7 [46][54].
2.3. Membrane Filtration
Membrane filtration is one of the most important physical methods for textile dye removal from wastewater [47][48][55,56]. In this method, due to the small pore size of the membrane, molecules larger than the filter pores become trapped. Microfiltration is a membrane-based phenomenon that involves the separation of dyes in the size range of 0.1–0.10 μm [49][57]. In this process, the waste materials or dyes are remediated from the liquid with the help of a microporous membrane. Ultrafiltration is another membrane-based method. Collivignarelli et al. [50][58] reported that dye color removal capabilities are acquired from wastewater with the help of ultrafiltration. The reactive black dye solution uses an ultrafiltration ceramic membrane and decolorizes the dye at different concentrations [51][59]. Reverse osmosis is also a membrane filtration mechanism that is used for the treatment of industrial wastewater that contains dyes [52][53][60,61]. Several researchers have reported the application of filtration technology for the treatment of wastewater containing dyes [54][55][56][62,63,64].
2.4. Fenton Process
The two major approaches for the degradation of dyes present in textile water are Fenton and photo-Fenton [57][58][65,66]. These processes are carried out by using a Fenton reagent, namely H2O2, and a soluble iron (II) salt mixture [59][60][61][67,68,69]. In a study by Ledakowicz et al. [62][70], it was reported that the degradation of three dyes (add red 27, reactive blue 81, and add blue 62) occurred with the use of a Fenton reagent. They concluded that this reaction is very simple and fast. In another study, Chen et al. [63][71] used the stopped flow technique to study the degradation of methylene blue and rhodamine B with Fenton reagent. Zawadzki and Deska [64][72] published a review concerning the degradation of dyes by combining advanced oxidation processes with different methods, such as utilizing ozone, hydrogen peroxide, and persulfate to degrade rhodamine B. They concluded that degradation is achieved by using UV in a photo-assisted ozonation, which was the most effective method among all of the techniques tested.
2.5. Ozonation
Dye remediation using ozonation is another important treatment technology. Shriram and Kanmani [65][73] concluded in their study that H2O2, UV radiation, etc., are used in the ozonation process. They provided a detailed study about the mechanism, influencing factors, and initiators of ozonation. Venkatesh and colleagues [66][74] reported dye remediation by means of combined ozonation and anaerobic treatment strategies. They reported that the cost of ozonation can be reduced using an upflow anaerobic sludge blanket reactor. In another study, Cardoso et al. [67][75] reported nanomaterial-based catalysts for the ozonation process in the remediation of dyes. They used copper (II)-doped carbon dots as catalysts in catalytic ozonation. They also analyzed the degradation of four dyes, namely methyl orange, orange II sodium salt, reactive black, and remazol brilliant blue R (RBB-R). In a recent research work, Lanzetta et al. [68][76] reported using the ozonation process for color remediation from tanning wastewater. Further research is needed on the decolorization of wastewater using ozonation.
3. Biological Approaches
The physical and chemical techniques used in dye decolorization are costly. Traditional biological techniques are used exclusively or with chemical and physical techniques for dye decolorization. Nonetheless, advanced biological methods that are less expensive and more effective with lesser secondary sludge production are emphasized [11][69][70][13,28,77]. Microbial methods are effective for the bioremediation of different types of organic and inorganic pollutants [27][71][72][30,78,79]. On the basis of different research findings, for the decolorization of dyes, bacterial treatment serves as an efficient strategy [73][80]. Figure 23 presents various biological methods for the bioremediation of dyes from contaminated environments.
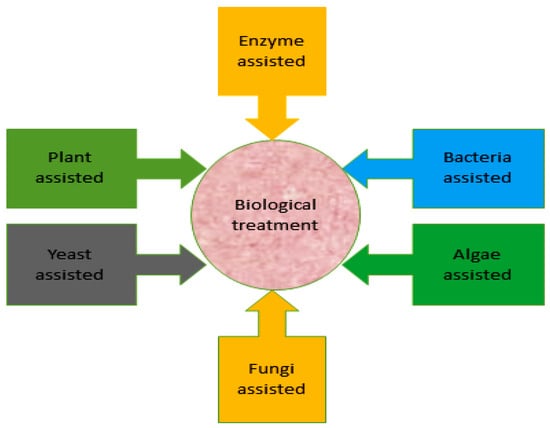

Figure 23.
Different strategies for the bioremediation of dyes from polluted environments.
3.1. Enzymatic Method
Bioremediation by enzymes is an ingenious, favorable, environmentally friendly technique [74][81]. The enzymatic degradation method consists of finding the attributes of microbes or genetically modified microbes, designing enzymes to metabolize the dyes, and transforming the harmful form of dyes into harmless forms or non-toxic forms [74][81]. Regarding azo dye degradation, a number of studies have been conducted to understand different type of enzymatic activities that help in the degradation of toxic dyes [74][75][81,82]. A class of enzyme, azoreductase, has been described by Mendes et al. [76][83] as carrying out the reduction reaction causing the breakdown of azo bonds (-N=N-) present in dyes, and converting the aromatic amine into colorless water. The enzyme laccase can be used in the treatment of different toxic textile dyes [77][84]. Lignin and Mn peroxidase have been widely studied; peroxidase enzymes have been used in the degradation of toxic textile dyes [78][85]. For the bioremediation of hydrocarbons and pesticides, enzymes produced by aerobic bacteria such as Alcaligene sp. and Pseudomonas sp. are also used [74][81]. Enzymes produced by different types of fungi, such as lignin peroxidases, azoreductases, and laccases from white rot fungi, can also take part in the biodegradation of textile dyes [79][86]. In a recently published review, Pinheiro et al. [80][87] showed the role of different enzymes in the bioremediation of dyes.
Various researchers such as Shahid et al. [81][88] found that the strain MR-1/2 of multifarious Bacillus species efficiently decolorized dyes such as reactive black-5, reactive red-120, direct blue-1, and Congo red, which additionally helped in the growth of the mung bean plant by alleviating azo dye toxicity. Meanwhile, Vineh et al. [82][89] found 100% decolorization of most of the dyes used in the study at pH 7, 25 °C, for 60 min by using peroxidase immobilized on a functionalized reduced grapheme oxide. In another study, Navas et al. [83][90] reported 20–100% decolorization of dyes at pH 5–9 using laccase, which was purified and extracted from the thermophilic Thermus species. In another study, Kalsoom et al. [84][91] found 95% degradation of remazol turquoise blue 133G dye with peroxidase from Brassica oleracea, while Gao et al. [85][92] achieved 72–80% decolorization of the azo dyes reactive blue 19 and acid orange 7 at a neutral to alkaline pH at relatively high temperatures using laccase enzymes immobilized in vault nanoparticles.
3.2. Microbial Remediation
Several microbes, such as bacteria, fungi, yeast, algae, and actinomycetes, are utilized for treating textile dyes from aquatic environments [3][8][86][87][88][89][90][6,10,93,94,95,96,97].
Bacterial Remediation
Among all the groups of microbes, decolorization by bacteria is significant. From a biotechnological perspective, bacteria offer many advantages as they contain abundant degradative enzymes, consequently having the capacity to degrade dyes of a broad range [3][91][92][6,98,99]. The basic advantage of dealing with bacteria is their efficiency in growing quickly, and their ability to be cultured easily. The organic pollutants that are aromatic hydrocarbon-based and chlorinated can be catabolized by bacteria as their source of energy [93][100]. Bacteria also have the capability to oxidize textile dyes based on sulphur to H2SO4 [94][101].
Pure Bacterial Cultures
Using a pure bacterial culture with one type of bacteria, the biodecolorization of dyes has been reported in several studies. In one investigation, Louati et al. [95][102], found 100% decolorization of dyes at pH 8 by using Pseudomonas aeruginosa strain Gb30, whereas Montanez-Barragan et al. [96][103] observed above 90% decolorization of dyes at pH 6–11 with Halomonas species. Another researcher, Shi et al. [97][104], found 100% dye removal of Brilliant Crocein by using the bacteria Providencia rettgeri, while Fareed et al. [98][105] found 80–100% decolorization of dyes by using free and immobilized cells of Bacillus cereus at temperatures of 32 °C, 37 °C, and 45 °C Srinivasan et al. [99][106] achieved 88.35–96.30% decolorization of different azo dyes, while Du et al. [100][107] observed the complete decolorization of malachite green and crystal violet at pH 3–10 and a temperature of 20–45 °C after 12 h of incubation under optimal environmental conditions using Serratia species, which is a new bacterial strain identified via 16S rDNA analysis. In a recent study, Tripathi et al. [8][10] observed 98% dye decolorization of crystal violet dye using a native multiple metal-tolerant Aeromonas caviae MT-1 isolate.
Mixed Bacterial Cultures
For decolorizing diverse groups of dyes, bacteria of a single species are not efficient enough for their remediation, and this is one of the most prominent challenges for environmental biotechnologists [101][108]. Many researchers have worked on a consortium or mixed bacterial culture for the degradation of dyes. In a study, Ayed et al. [102][109] observed 90% dye decolorization at 35 °C using a bacterial consortium consisting of Sphingomonas paucimobilis, Pseudomonas putida, and Lactobacillus acidophilus. In another study, Guo et al. [103][110] found 93% decolorization at 40 °C and pH 10 of Methanil Yellow G dye using a consortium with Halomonas, Marinobacter, and Clostridii salibacter., Meanwhile, in another investigation, Joshi et al. [101][108] reported dye decolorization of 24–94% using a consortium of six bacterial strains: Pseudomonas stutzeri AK1, P. stutzeri AK2, P. stutzeri AK3, Bacillus sp. AK4, P. stutzeri AK5, and P. stutzeri AK6. Likewise, Bera et al. [104][111] reported 85% acid orange dye decolorization after nearly 23 h, with yeast as a supplementary source and a bacterial consortium called novel bacterial consortium SPB92 composed of four bacterial strains, i.e., Pseudomonas stutzeri (MW219251), Bacillus tequilensis (MW110471), B. flexus. (MW13 flexus and Kocuria rosea (MW 132411), whereas Neihsial et al. [105][112] found 85–97% degradation of dyes using a consortium including bacteria of different genera, like Acinetobacter, Comamonas, Trichococcus, Erwinia, Dysgonomonas, and Citrobacter. In another study, Barathi et al. [106][113] found that the bacterial consortium with three bacterial species, B. subtilis, Brevibacillus borstelensis, and B. firmus, was able to degrade dye at a better rate at lower concentrations of dye, but the ability of degradation decreased with elevated concentrations of dye. Several bacterial isolates have been reported for dye decolorization.
Actinomycetes
Microorganisms, particularly actinomycetes, play a significant role in the decolorization of dyes. Several researchers have reported the decolorization of dyes using actinomycetes. Zhou and Zimmermann [90][97] observed 3–10% decolorization of dyes by Streptomyces species. On the other hand, in recent research, Dong et al. [107][119] observed 99% decolorization of dyes using Streptomyces sp. S27. In another study, Adenan et al. [108][120] observed 64–94.7% decolorization of different dyes using actinomycetes. They reported the decolorization of triphenylmethane dyes using Streptomyces bacillaris through biosorption and biodegradation mechanisms. Raja et al. [109][121] studied the decolorization of amido black dye by means of actinomycetes isolated from marine sediments under aerobic conditions. They found significant 88% decolorization at a 5 ppm dye concentration. Meanwhile, Blánquez et al. [110][122] observed decolorization of 6–70% using the actinomycetes Streptomyces ipomoeae CECT 3341. In another study, Kameche et al. [111][123] studied the decolorization of the azo dye Evans blue using four strains of Streptomyces isolated from soils. They observed a 97% remediation rate at an initial 50 mg/L Evans blue concentration.
Phycoremediation
Studies suggest that azo dyes are utilized as a carbon and energy source for algae, which are then degraded into aromatic amines, subsequently being converted into simple inorganic and organic compounds [112][124]. For the investigation of dye decolorization from textile wastewater samples, Chlorella species, Oscillatoria species, Phormidium species, and Synechocystis have been widely used [113][125]. Many functional groups like carboxy, carbonyl, hydroxy, phosphoryl, and amide groups are present in algal cell walls, which help in dye decolorization [114][126]. In another investigation, Mahajan et al. [115][127] reported 70–100% decolorization of methyl red dye at pH 3.5–9.5 using Chara vulgaris L. In another study by Boulkhessaim et al. [116][128], they reported 45–80% decolorization of dyes using Chlorella vulgaris, whereas Dellamatrice et al. [117][129] observed 91% dye decolorization using Cyanobacterium phormidium. In another study, Alprol et al. [118][130] found 75.7% and 61.11% decolorization of dyes by using Arthrospira platensis complete dry biomass and lipid-free biomass. Meanwhile, Mansour et al. [119][131] reported 93% decolorization of methylene blue using A. platensis. These studies indicate that algae application for effective remediation of dye pollution is a viable and eco-friendly option for a sustainable and green environment.
Yeast-Mediated Dye Decolorization
Yeast has not been as thoroughly studied and used in the decolorization of dyes as filamentous fungi and bacteria [120][132]. The removal of dyes using yeast was reported in the biosorption process [120][132]. The decolorization of azo dye through yeasts is achieved using the enzyme azoreductase present in yeast [121][133]. Galactomyces geotrichum MTCC1360 has the capacity to decolorize azo dyes [122][134]. Researchers like Guo et al. [123][135] reported 92% decolorization of the azo dye Acid Scarlet GR using the newly isolated salt-tolerant yeast strain Galactomyces geotrichum GG. In another study, Ali et al. [124][136] reported 82% decolorization of lignin-like dyes and wastewater containing textile dyes using a recently formed oleaginous yeast consortium with three yeast cultures: Yarrowia sp. SSA1642, Barnettozyma californica SSA1518, and Sterigmatomyces halophilus SSA1511.
Phytoremediation
Phytoremediation involves the plant-mediated treatment of contaminants from the environment, and it is a cost-effective solution for the clean-up of various types of pollutants [125][126][137,138]. This process operates through several mechanisms or processes to remediate contaminants. The different phytoremediation strategies, such as phytoextraction (uptake or absorption of contaminants by roots into the shoots for metabolization), phytostabilization (compounds secreted by the plant immobilize contaminants rather than degrade them), phytovolatilization (involves translocation of contaminants by roots to aerial plant parts where they volatilize into the atmosphere), and rhizofiltration (plant roots absorb the contaminants, which are then metabolized or stored), are widely used, and accepted as a cost-effective environmental restoration technology [127][128][129][130][139,140,141,142].
In phytoremediation, plants interact at the physical, chemical, biological, and microbial levels to reduce pollutant toxicity. This employs a variety of processes depending on the form and quantity of the pollutant [128][140]. In their investigation, Biju et al. [131][143] found 75 ± 0.5% and 82 ± 0.5% decolorization of a mixture of azo dyes using Salvinia species. Rane et al. [132][144] observed complete decolorization of sulfonated remazol red dye and effluents of the textile industry using Alternanthera philoxeroides. In another study, Imron et al. [133][145] found the decolorization of 80.56 ± 0.44% of methylene blue dye using duckweed (Lemna minor), whereas Baldawi et al. [134][146] observed 85% decolorization of methylene blue dye using the floating plant Azolla pinnata.
