Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 3 by Jason Zhu.
The functionalization of unactivated substrates through the combination of copper catalysts and hypervalent iodine reagents represents a versatile tool in organic synthesis to access various classes of compounds. The hypervalent iodine derivatives can be used simply as oxidizing agents to regenerate the catalytic species or they can associate the functionalization of the starting material.
- copper catalysis
- hypervalent iodine
- difunctionalization reactions
1. Introduction
Copper-catalyzed oxidative coupling reactions involving multiple unactivated C-C bonds have emerged as an important strategy for rapidly increasing molecular complexity in organic synthesis. Compared to the classic coupling reactions, this approach avoids the need for pre-functionalized reactants, even if the success of the course depends on the ability of an appropriate oxidizing agent to restart the catalytic cycle. In this context, the hypervalent iodine reagents have gained a prominent role due to their unique features as non-toxic, relatively cheap, easy to handle, readily available, and with low environmental impact compared to the toxic heavy metal-based oxidants and expensive organometallic catalysts.
Today, hypervalent iodine compounds occupy a leading role in organic synthesis since they can be used as efficient reagents or simply as oxidizing agents [1][2][3][4][5][6][7][8][9][10][11][12]. Organic derivatives of iodine in the (III) and (V) oxidation states are usually used in organic synthesis as reagents for various selective oxidative transformations of complex organic molecules.
Hypervalent iodine reagents make the approach to organic synthesis more flexible as they allow the reversal of the normal polarity of functional groups, leading to their umpolung. They behave as electrophilic synthons, accessible from nucleophiles by exploiting the electrophilicity of the iodine atom and the reactivity of the hypervalent bond. Since the publication of the pioneering works in this field, both iodine and iodonium salts have assumed a privileged position for the possibility of transforming many nucleophiles into electrophilic synthons. However, although highly reactive, they can be unstable in the presence of strong bases, transition metals or heating, becoming unsuitable for many applications. To overcome this drawback, hypervalent cyclic iodine reagents are used, as the inclusion of the iodine atom in a heterocycle has shown greater stability than the acyclic equivalents [13][14]. As a mild oxidizing agent, Dess-Martin periodinane is used for the conversion of alcohols to aldehydes and ketones [15][16][17][18], while 2-iodoxybenzoic acid allows the oxidation of the benzylic position or the introduction of α,β-double bonds in carbonyl compounds [19][20]. The excellent reactivity of iodine(III) compounds due to their electrophilic character has been exploited to carry out alkynylation, azidation, cyanation and trifluoromethylation processes, also on the basis of the further opportunity to modulate the reactivity through the trans-effect in the hypervalent bond [21]. The availability of ethinyl [22][23], azido [24] and cyano-benziodoxolones [25] (EBX, EBX, and CBX, respectively) has allowed reactions of multifunctionalization to develop with the formation of more than one bond in a single transformation. Particularly interesting are the intramolecular oxidative transition metal-catalyzed processes of C-H functionalization that allow to associate the cyclization by alkoxylation and amination of multiple carbon–carbon bonds with the introduction of functional groups which provide direct access to functionalized heterocyclic systems. Conversely, diaryliodonium reagents have been recognized as efficient alternatives to transition metals. The most important and widespread application in the field of organic synthesis concerns arylation reactions using diaryliodonium salts. Due to the highly electron-deficient nature of diaryliodonium salts at the iodine core and the excellent behaviour as the leaving group of iodobenzene, they serve as versatile arylating agents with a variety of nucleophiles. Diaryliodonium salts with a halide anion are poorly soluble in most of the organic solvents, while triflates and tetrafluoroborates have better solubility.
2. Hypervalent Iodine as Reagent in C-C Bond Formation
2.1. Arylation Reactions
Biaryl and aryl-heteroaryl moieties are ubiquitous substructures in several compounds of primary interest in organic chemistry. Thus, arylation chemistry always demands new direct and selective procedures, possibly involving C-H bonds [26][27][28]. The combination of a copper catalyst and aryl hypervalent iodine derivatives was proven to be an innovative tool for the unusual meta-selective C–H bond arylation of electron-donor substituents in aromatic rings. The presence of a suitable carbonyl group as a directing group is essential to achieve meta-selectivity. In 2009, Gaunt’s group described the meta-arylation of pivanilides combining the use of catalytic Cu(OTf)2 with Ph2IOTf, Ph2IBF4 or [Mes-I-Ar]OTf (Scheme 1) [29]. The proposed mechanism, also studied theoretically by DFT calculations [30], involves the high electrophilic aryl-Cu(III) specie I as the key intermediate.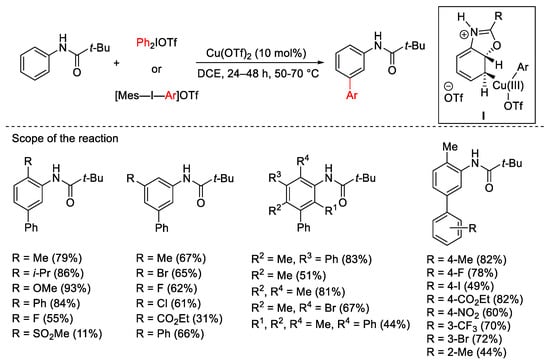
Scheme 1. Cu(OTf)2-catalyzed arylation of pivanilides.
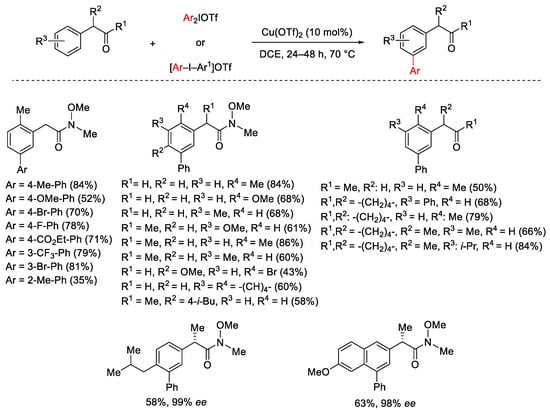
Scheme 2. Cu(OTf)2-catalyzed arylation of α-aryl-substituted carbonyl compounds.
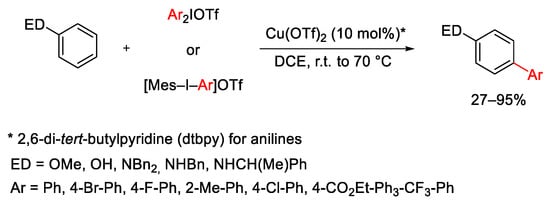
Scheme 3. Cu(OTf)2-catalyzed arylation of electron-rich arenes.
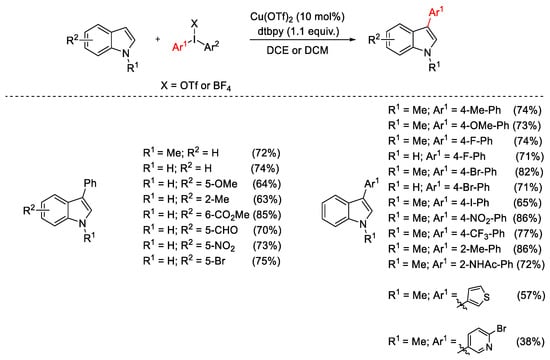
Scheme 4. Cu(OTf)2-catalyzed C3-arylation of indoles.
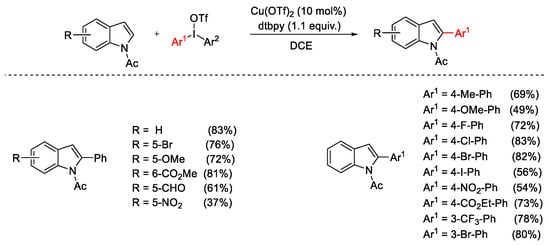
Scheme 5. Selective Cu(OTf)2-catalyzed C2-arylation of N-acetylindoles.
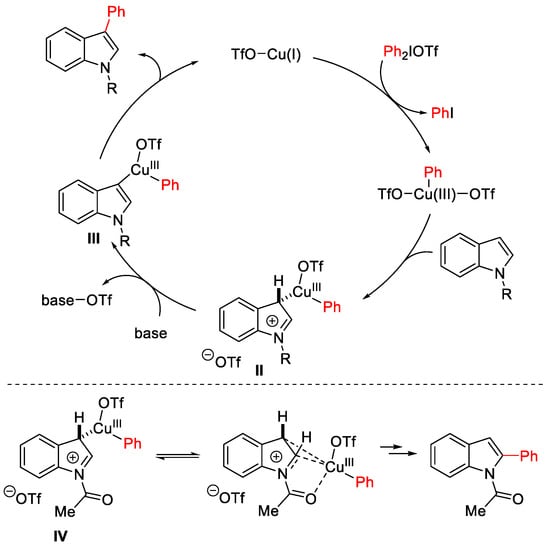
Scheme 6. Proposed mechanism for the selective Cu(OTf)2-catalyzed C3 arylation of indoles vs. C2-arylation of N-acetylindoles.
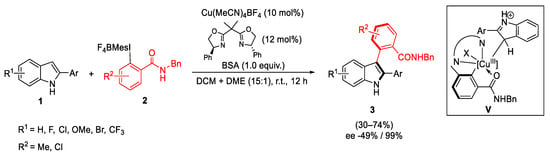
Scheme 7. Atroposelective synthesis of 2,3-diarylindoles.
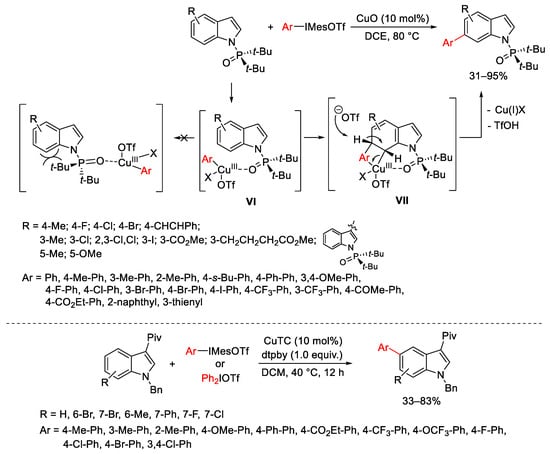
Scheme 8. Selective C5- and C6-arylation of substituted indoles.
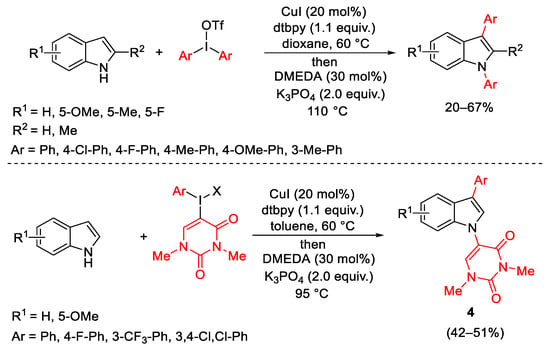
Scheme 9. Tandem diarylation of indoles.
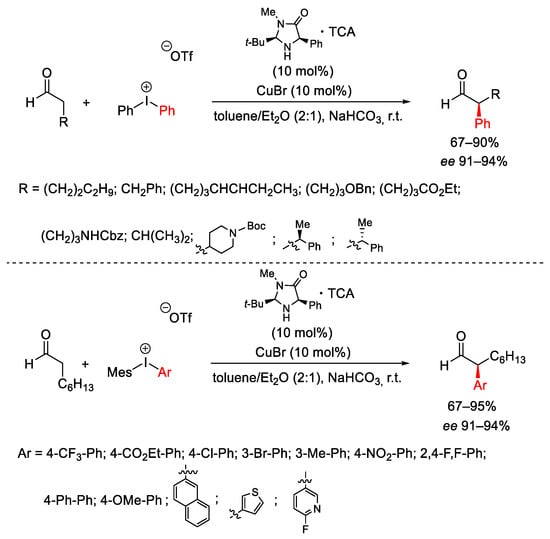
Scheme 10. Stereoselective arylation of enolizable aldehydes.
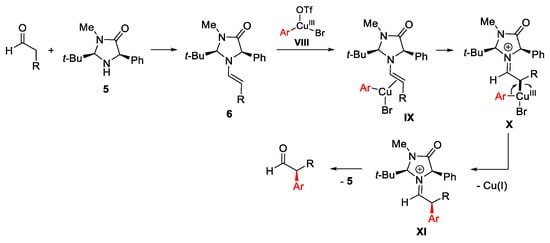
Scheme 11. Proposed mechanism for stereoselective arylation of enolizable aldehydes.
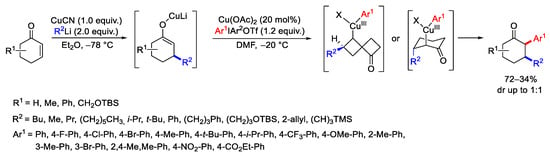
Scheme 12. Synthesis of α-aryl-β-substituted cyclic ketones.
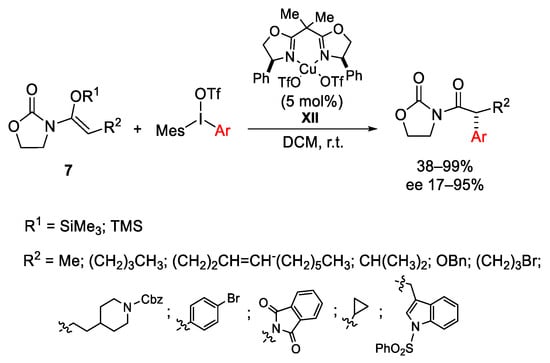
Scheme 13. Enantioselective α-arylation of N-acyloxazolidinones.
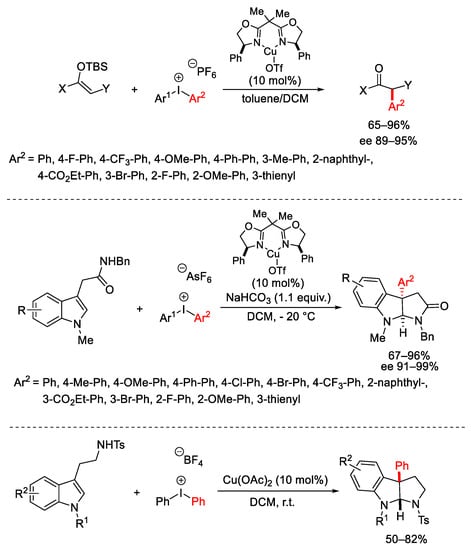
Scheme 14. α-Arylation of enolsilanes and indole 3-acetamides.
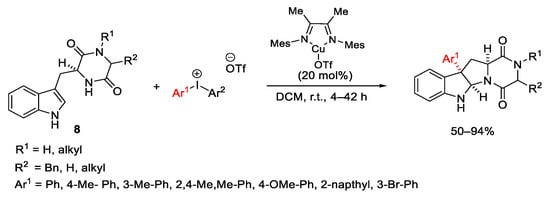
Scheme 15. Diastereoselective arylation of tryptophan derivatives.
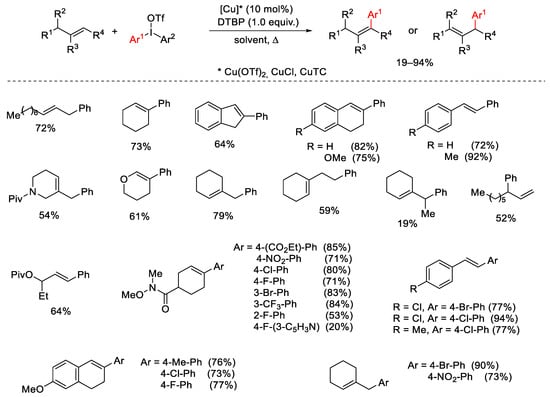
Scheme 16. Copper-catalyzed arylation of alkenes with diaryliodonium salts.
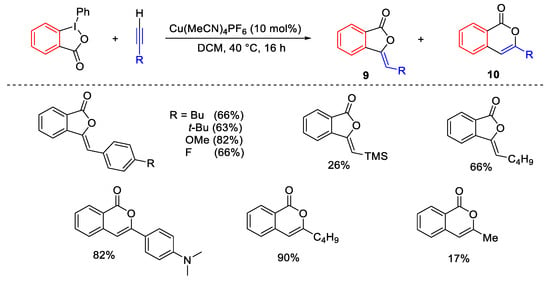
Scheme 17. Access to phthalides and isocumarins from terminal alkynes.
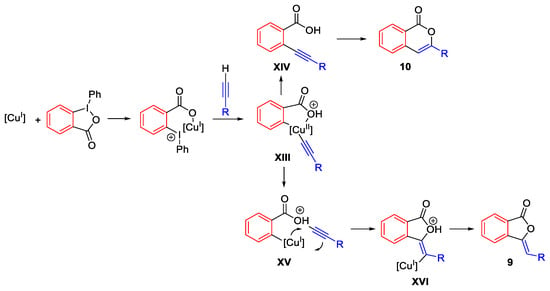
Scheme 18. Proposed mechanism for the conversion of terminal alkynes into phthalides and isocumarins.
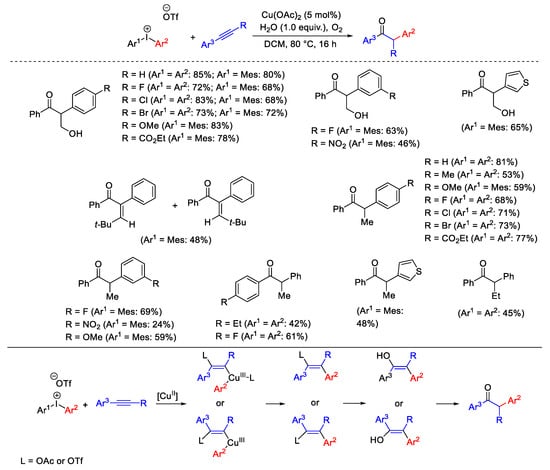
Scheme 19. Arylation reaction of internal alkynes with diaryliodonium salts to α-arylketones.
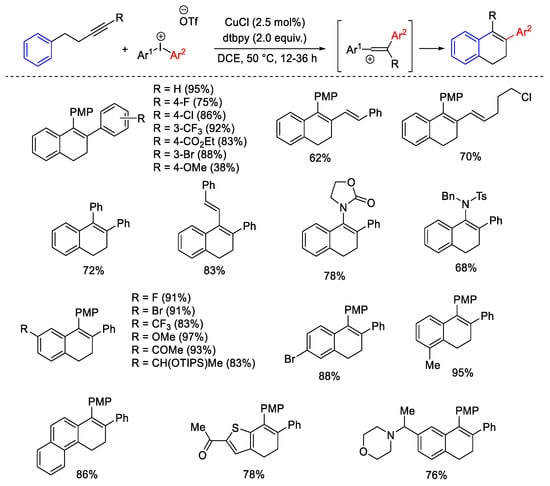
Scheme 20. Synthesis of dihydronaphthalene scaffold from arylalkynes and diaryliodonium salts in the presence of CuCl catalyst and dtbpy.
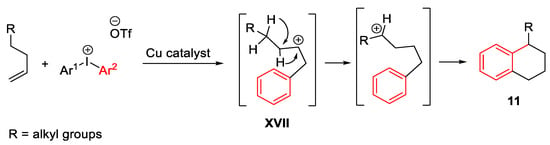
Scheme 21. Copper-catalyzed arylation of alkenes with diaryliodonium salts.
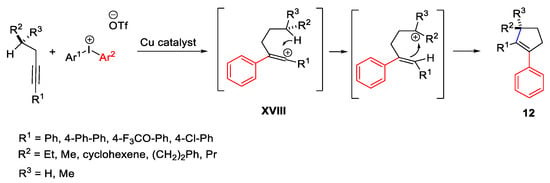
Scheme 22. Copper-catalyzed arylation of alkynes with diaryliodonium salts.
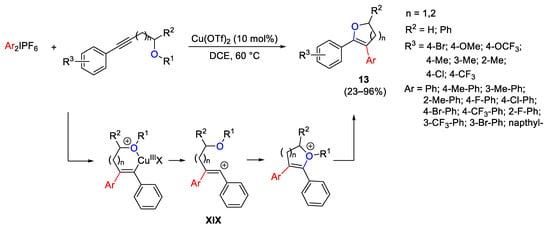
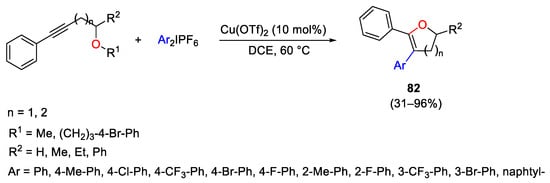
Scheme 23. Synthesis of oxygen-containing heterocycles from alkoxyalkynes and diaryliodonium salts.
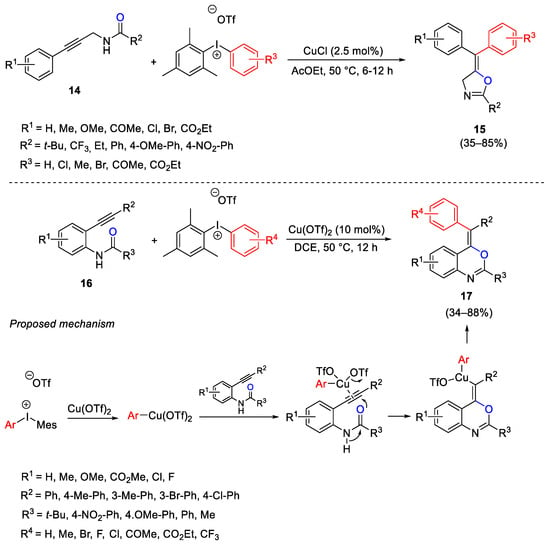
Scheme 24. Carboarylation of propargylamides and 2-ethynylanilides for the construction of oxazolines and benzoxazines.
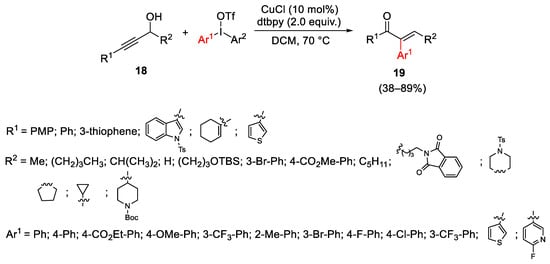
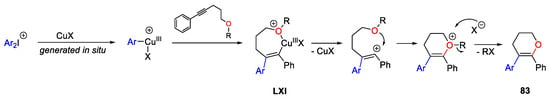
Scheme 25. Copper-catalyzed Meyer–Schuster rearrangement of propargyl alcohols.
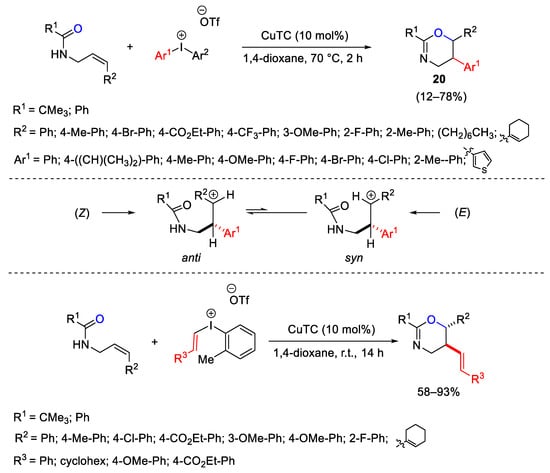
Scheme 26. Oxyarylation and oxyvinylation of allyl amides with diaryl- and vinyl(aryl)iodonium triflates.
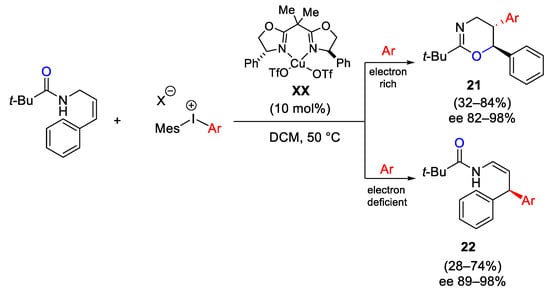
Scheme 27. Copper-catalyzed arylation of allylamides dependent on the electronic properties of diaryliodonium salts.
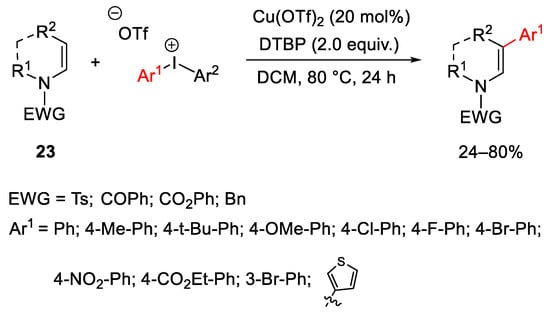
Scheme 28. Cu(OTf)2-catalyzed β-arylation of enamides.
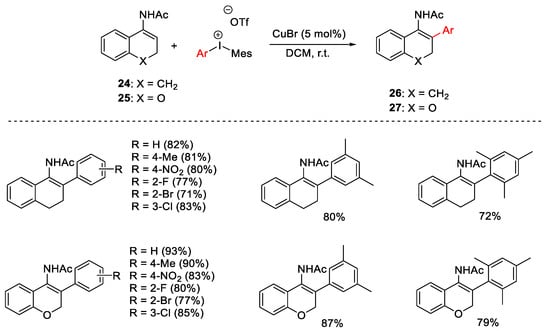
Scheme 29. β-Arylation of secondary cyclic enamides.
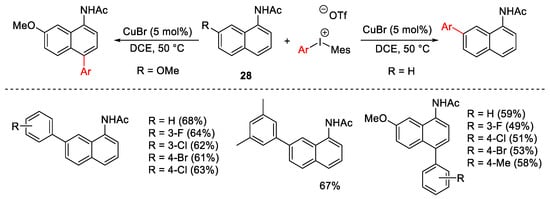
Scheme 30. C4 and C7 arylation of naphthyl-1-acetamides.
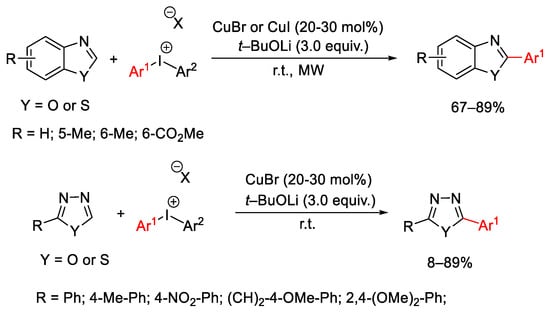
Scheme 31. C-H arylation of aza-heterocycles.
2.2. Alkynylation Reactions
Oxy-alkynylation of diazocompounds with ethynylbenziodoxolone (EBX) involving the formation of carbene intermediate was reported by the Waser group. The mild reaction conditions allowed broad applicability to diazocompounds and many functional groups are tolerated. The obtained products 29 and 30 were easily transformed into building blocks such as secondary and tertiary propargylic hydroxy acids, triazoles and isocoumarins (Scheme 32) [61].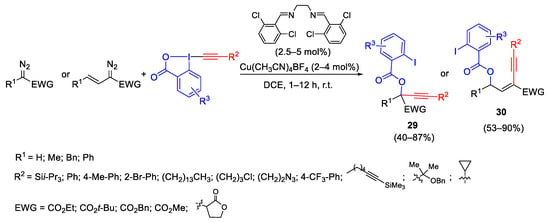
Scheme 32. Copper-catalyzed oxy-alkynylation of diazo compounds with EBX.
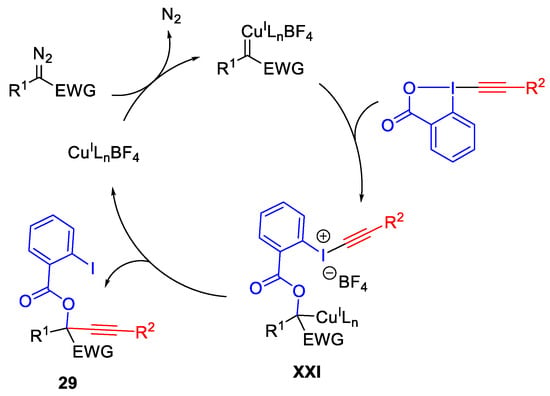
Scheme 33. Proposed mechanism for oxy-alkynylation.
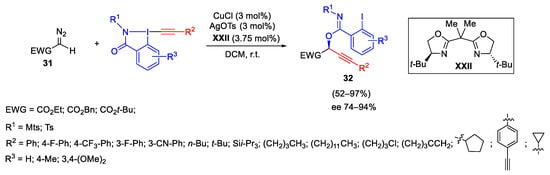
Scheme 34. Copper-catalyzed enantioselective oxyalkynylation of diazo compounds.
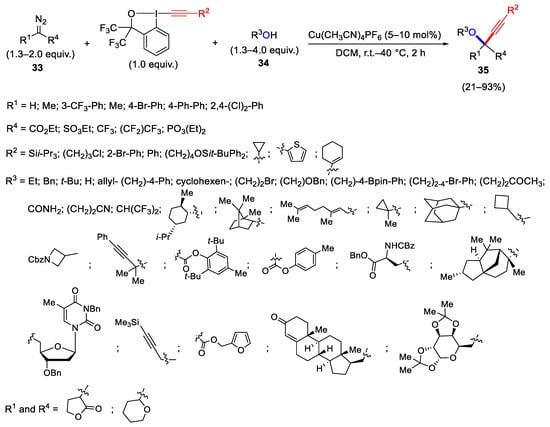
Scheme 35. Synthesis of propargyl ethers through three-component oxyalkynylation reaction.
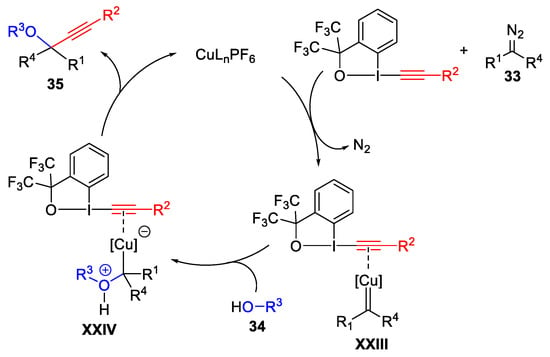
Scheme 36. Proposed mechanism for the three-component oxyalkynylation reaction.
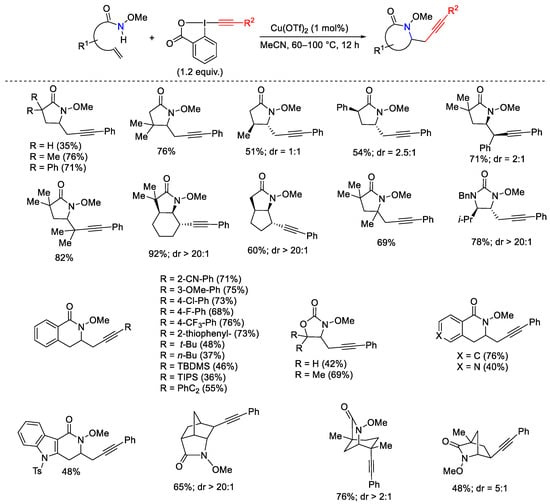
Scheme 37. Cu-catalyzed aminoalkynylation of alkenes with EBX.
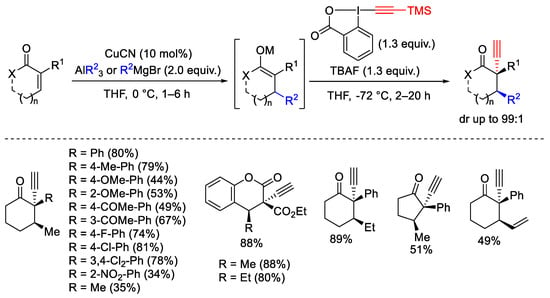
Scheme 38. Sequential Michael addition/electrophilic alkynylation on α,β-unsaturated cyclic ketones.
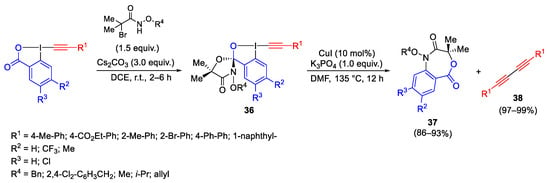
Scheme 39. Synthesis and reactivity of cyclic λ3-iodanes.
2.3. Trifluoromethylation Reactions
Among the new methods for the introduction of trifluoromethyl group into a variety of organic molecules, the use of hypervalent iodine reagents under copper catalysis was of significance for the efficiency of the reaction. Two are the useful hypervalent iodine compounds containing the CF3 group, the Togni reagents 1(I) and 2(II), reported for the first time by Togni’s research group in 2006 (Scheme 40).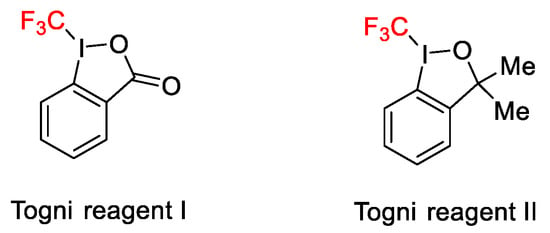
Scheme 40. Togni’s reagents.
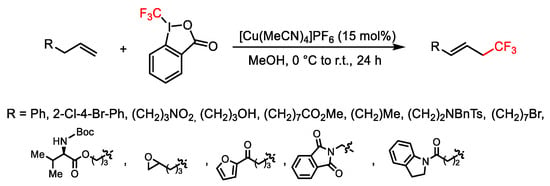
Scheme 41. Trifluoromethylation of terminal alkenes.
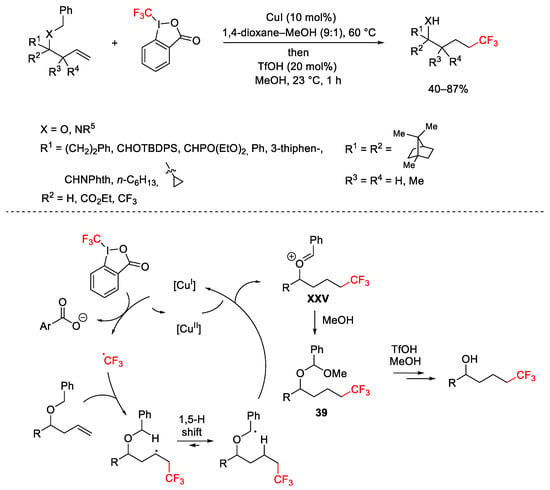
Scheme 42. Trifluoromethylation of homoallylic alcohol derivatives.
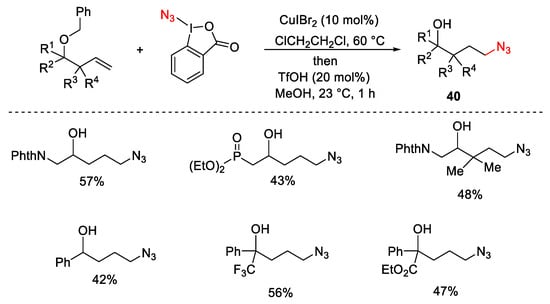
Scheme 43. Formation of azidoalcohols.
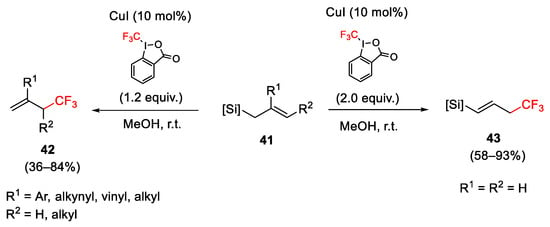
Scheme 44. Trifluoromethylation of allylsilanes with Togni reagent I.
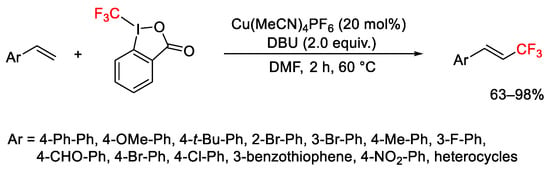
Scheme 45. Trifluoromethylation of styrenes.
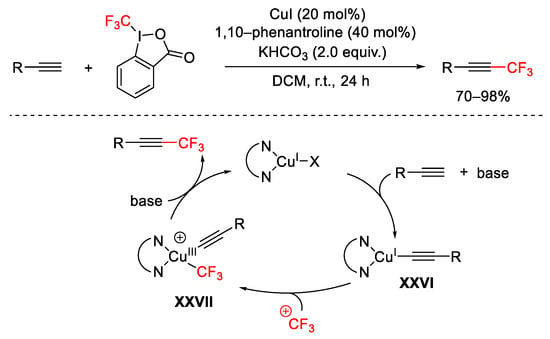
Scheme 46. Trifluoromethylation of terminal alkynes.
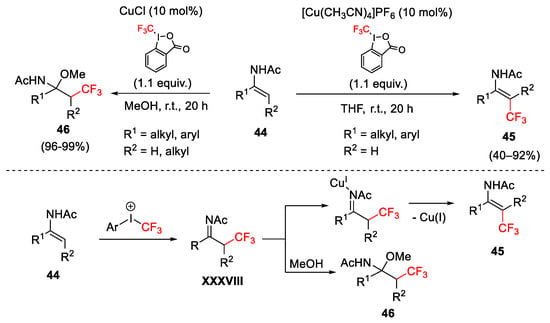
Scheme 47. Trifluoromethylation of electron-rich enamides.
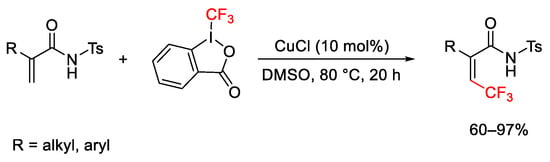
Scheme 48. Trifluoromethylation of electron-deficient alkenes.
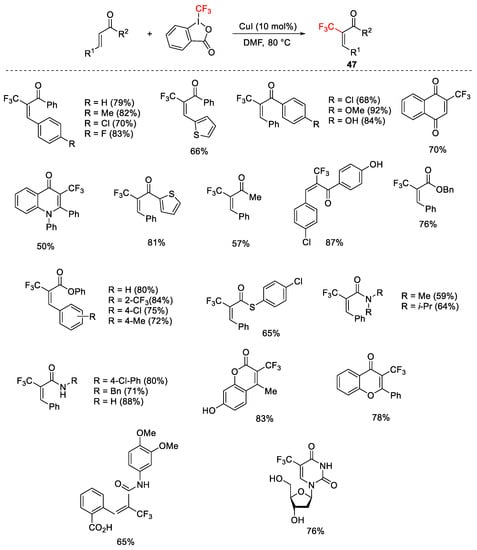
Scheme 49. Trifluoromethylation of α,β-unsaturated carbonyl compounds.
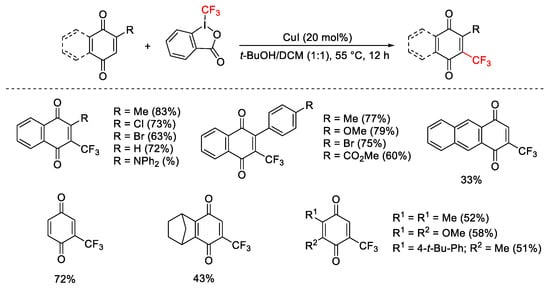
Scheme 50. Trifluoromethylation of quinones.
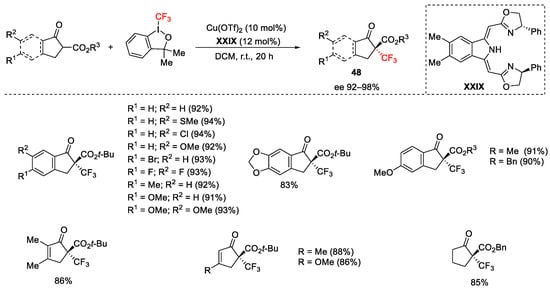
Scheme 51. Trifluoromethylation of β-ketoesters.
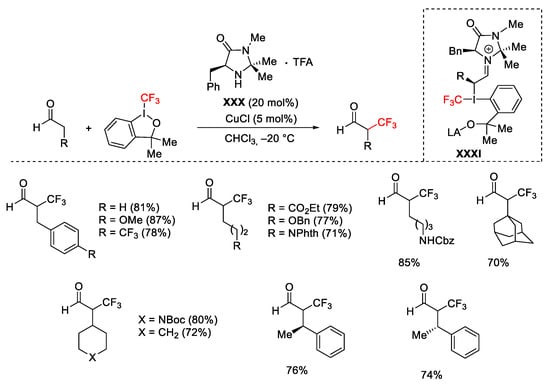
Scheme 52. Enantioselective α-trifluoromethylation with organocatalyst.
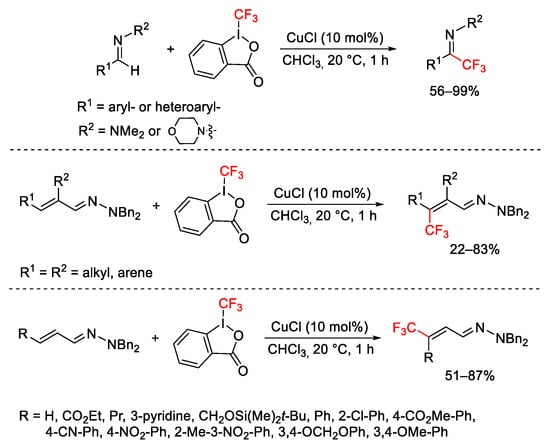
Scheme 53. Copper-catalyzed trifluoromethylation of hydrazones.
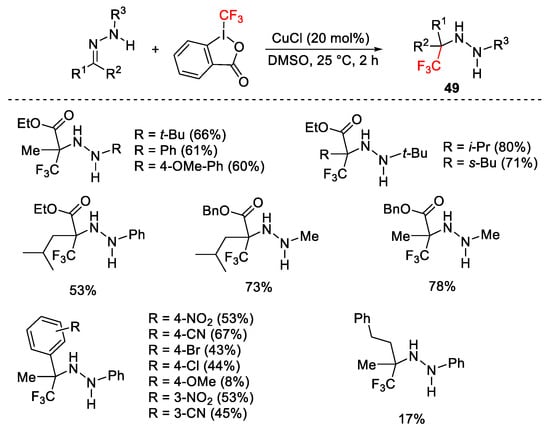
Scheme 54. Trifluoromethylation of N-monosubstituted hydrazones.
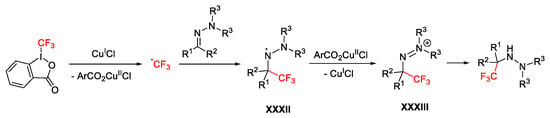
Scheme 55. Mechanism for the trifluoromethylation of hydrazones.
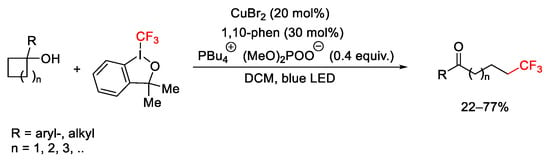
Scheme 56. Terminal trifluoromethylation of ketones by C-C bond cleavage of cycloalkanols.
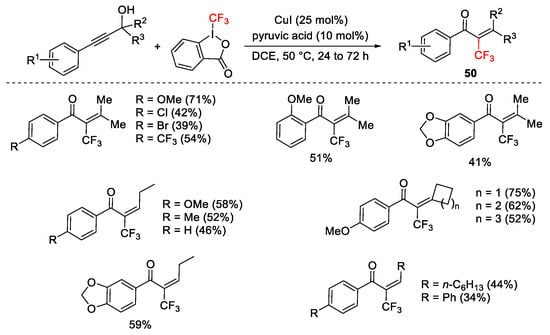
Scheme 57. Copper-catalyzed domino trifluoromethylation/Meyer–Schuster rearrangement.
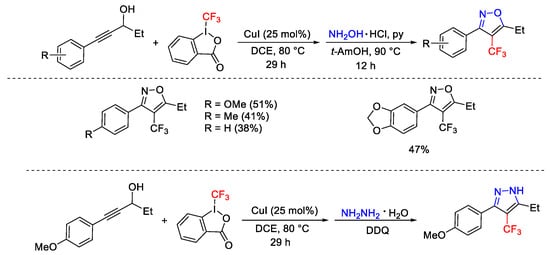
Scheme 58. Copper-catalyzed domino trifluoromethylation/Meyer–Schuster rearrangement.
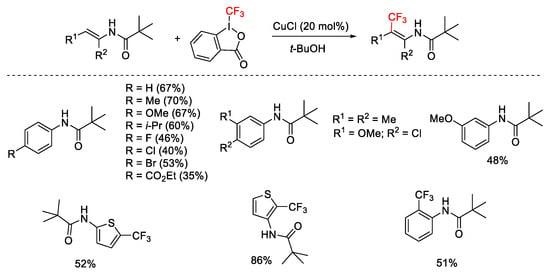
Scheme 59. Copper-catalyzed trifluoromethylation of (hetero)arenes with Piv as directing group.
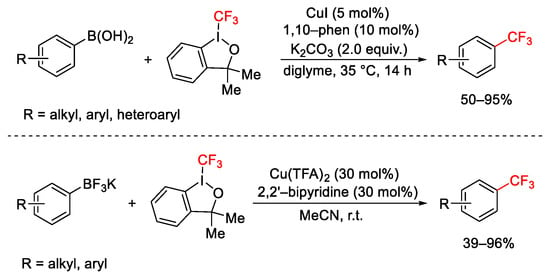
Scheme 60. Trifluoromethylation of organo boronic acids and organotrifluoroborates.
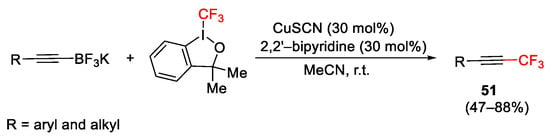
Scheme 61. Trifluoromethylation of alkynyltrifluoroborates.
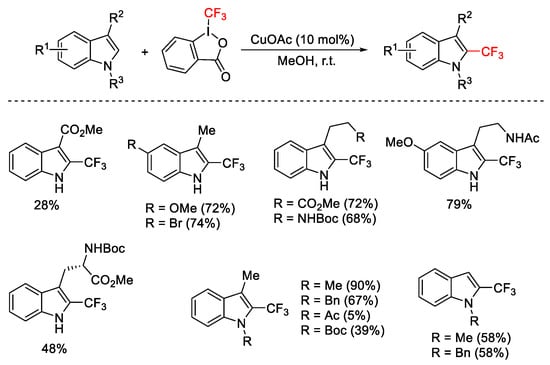
Scheme 62. Trifluoromethylation of C2 position of indoles.
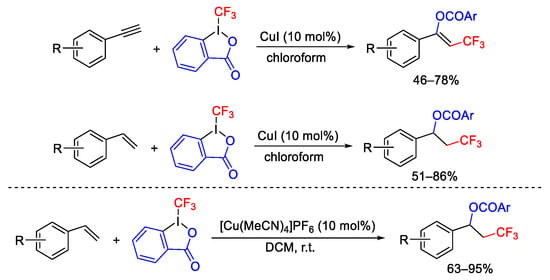
Scheme 63. Oxytrifluoromethylation of alkenes and alkynes.
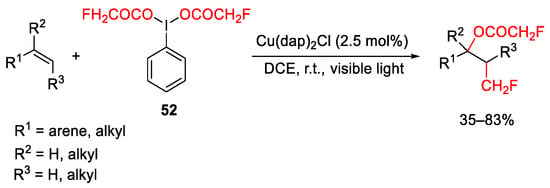
Scheme 64. Copper-catalyzed oxy-monofluoromethylation of alkenes.
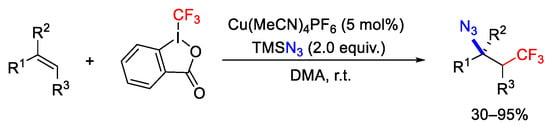
Scheme 65. Copper mediated trifluoromethylazidation of alkenes.
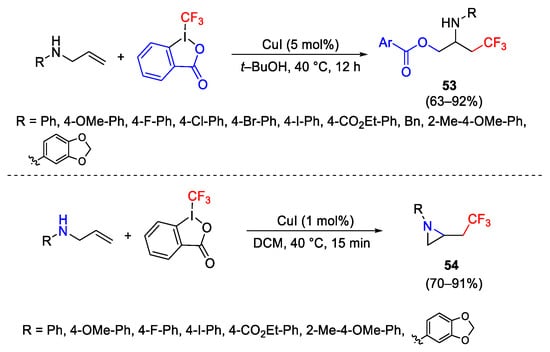
Scheme 66. Synthesis of β-trifluoromethylamines.
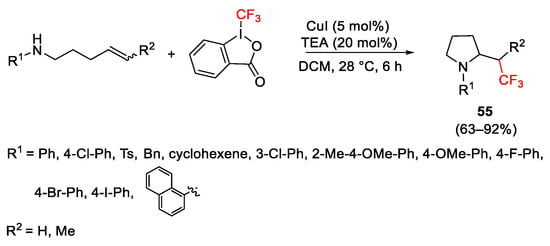
Scheme 67. Synthesis of β-trifluoromethylamines.
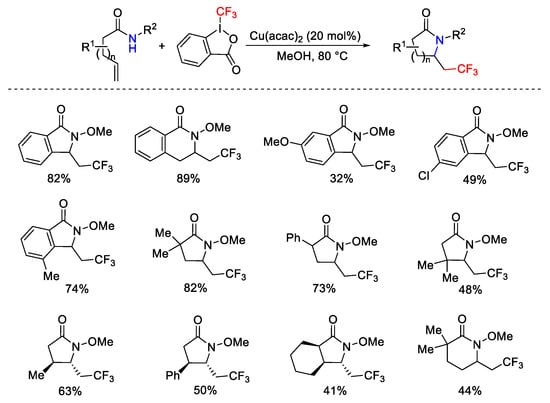
Scheme 68. Synthesis of CF3-containing lactams.
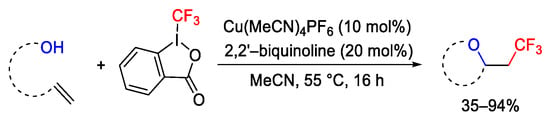
Scheme 69. Oxytrifluoromethylation of unactivated alkenes.
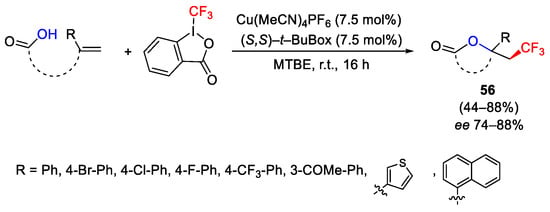
Scheme 70. Asymmetric oxytrifluoromethylation of alkenes.
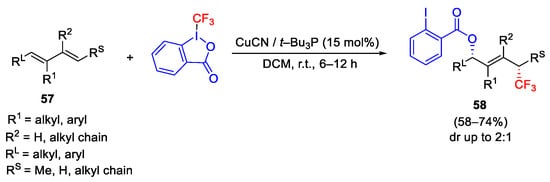
Scheme 71. Copper-catalyzed acyloxytrifluoromethylation of dienes.
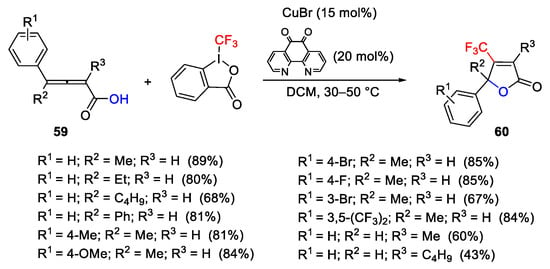
Scheme 72. Copper-catalyzed oxytrifluoromethylation of 2,3-allenoic acids.
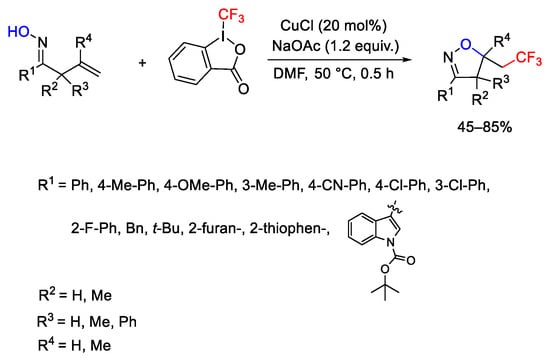
Scheme 73. Copper-catalyzed synthesis of trifluoromethyl-substituted isoxazolines.
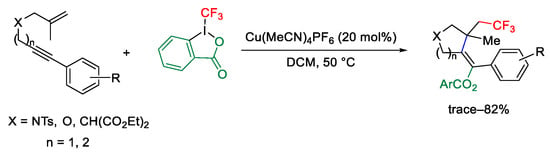
Scheme 74. Copper-catalyzed trifluoromethylation/cyclization of enynes.
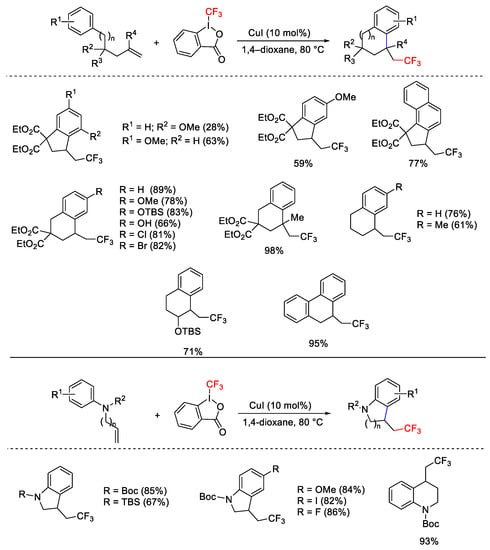
Scheme 75. Domino C-C and C-CF3 bond formations.
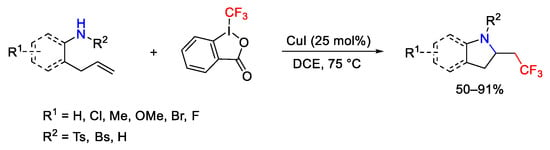
Scheme 76. Aminotrifluoromethylation of unactivated alkenes.
3. Hypervalent Iodine as Reagent in C-N Bond Formation
3.1. Arylation Reactions
Diaryliodonium salts have been employed as arylating agents of a wide range of nitrogen nucleophiles under copper(I)-catalyzed conditions. The most general and accepted mechanism, depicted in Scheme 77, occurs through oxidative addition of the diaryliodonium salt with the Cu(I)-catalyst forming the electrophilic Cu(III)-intermediate XXXIV. Coordination of the nitrogen nucleophile generates XXXV which in turn, in the presence of a base, undergoes reductive elimination giving the target product with regeneration of the Cu(I)-catalyst.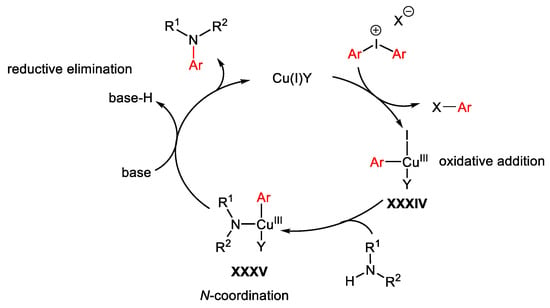
Scheme 77. General mechanism for Cu-catalyzed C-H amination reactions in the presence of hypervalent iodine reagents.
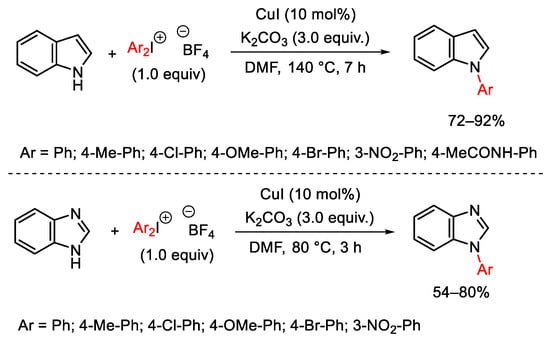
Scheme 78. Copper(I)-catalyzed arylation of indole and benzimidazole.
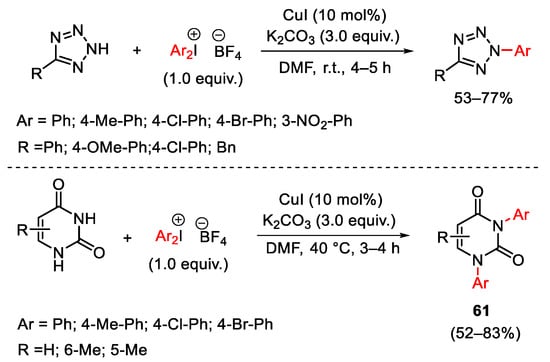
Scheme 79. Copper(I)-catalyzed arylation of tetrazole and uracil derivatives.
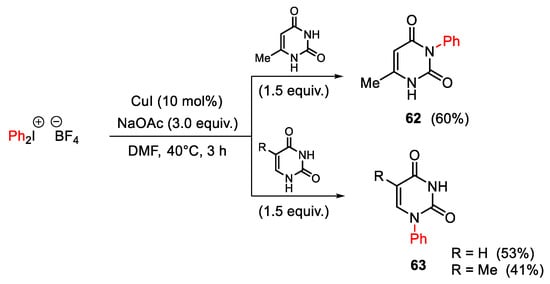
Scheme 80. Regioselective copper(I)-catalyzed arylation of 6-methyluracile, thymine and uracil.
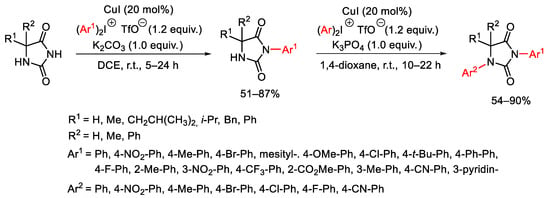
Scheme 81. Copper(I)-catalyzed arylation of hydantoins.
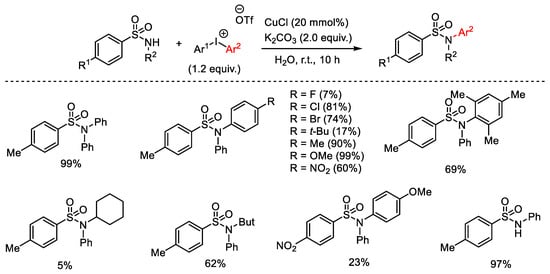
Scheme 82. Copper(I)-catalyzed arylation of sulfonamides with diaryliodonium salts.
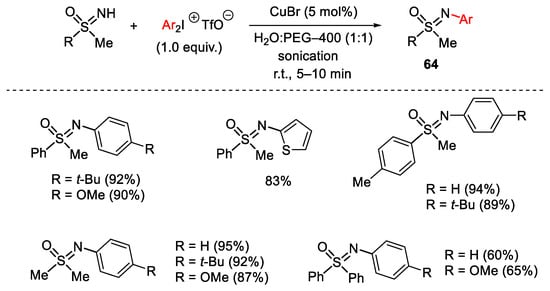
Scheme 83. Copper(I)-catalyzed arylation of sulfoximines with diaryliodonium salts.
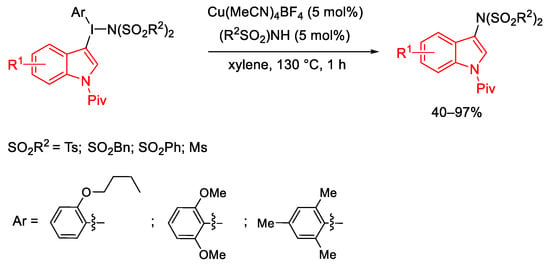
Scheme 84. Copper(I)-catalyzed C-N coupling reaction using indolyl(aryl)iodonium imides.
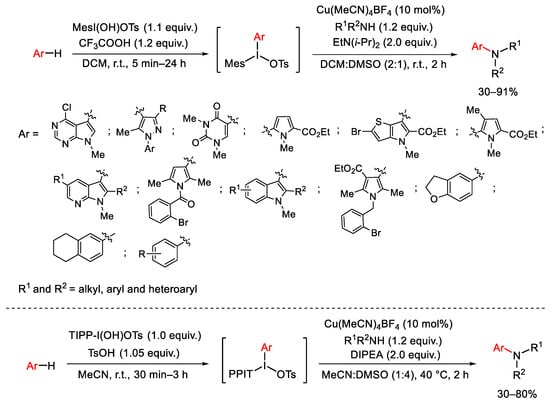
Scheme 85. Copper(I)-catalyzed C-H amination of unsymmetric diaryliodonium salts with amine compounds.
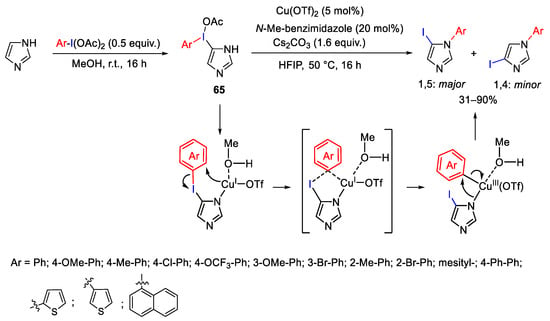
Scheme 86. Copper catalyzed aryl migration: selective synthesis of N-aryl-5-iodoimidazoles.
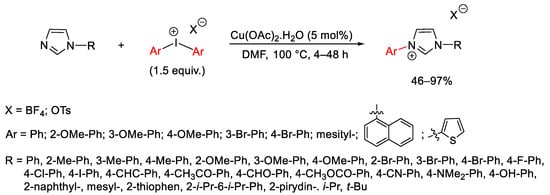
Scheme 87. Aryl quaternization of imidazole derivatives under copper catalysis.
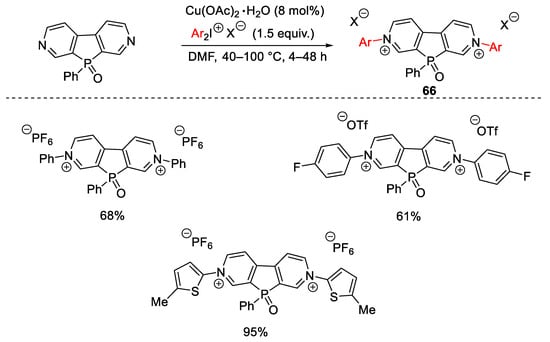
Scheme 88. Aryl quaternization of electron-deficient pyridines under copper catalysis.
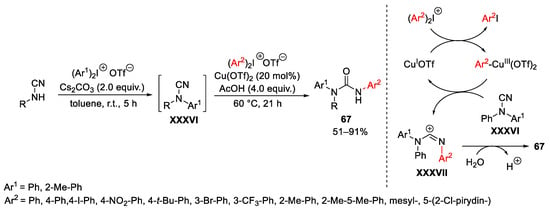
Scheme 89. Copper-catalyzed synthesis of unsymmetrical diarylureas.
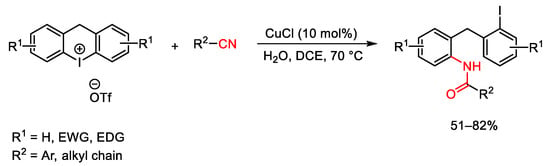
Scheme 90. Copper-catalyzed synthesis of diarylmethane amides using cyclic iodonium salts.
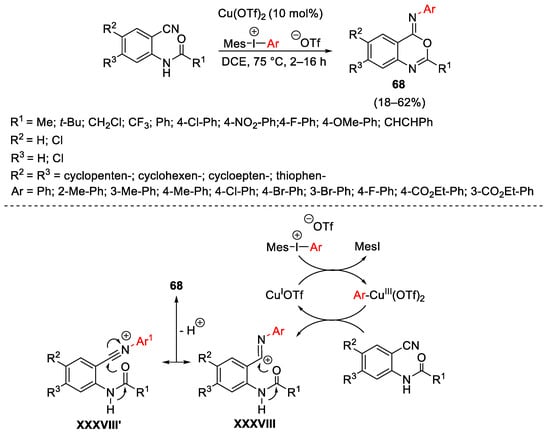
Scheme 91. Copper-catalyzed ring closure of o-cyanoanilides with hypervalent iodine reagents.
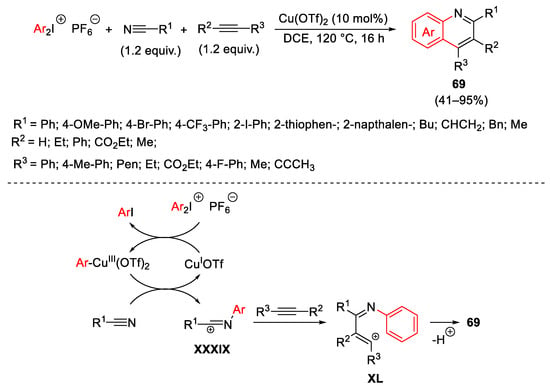
Scheme 92. Copper-catalyzed synthesis of substituted quinolines 69 by a three-component cascade annulation.
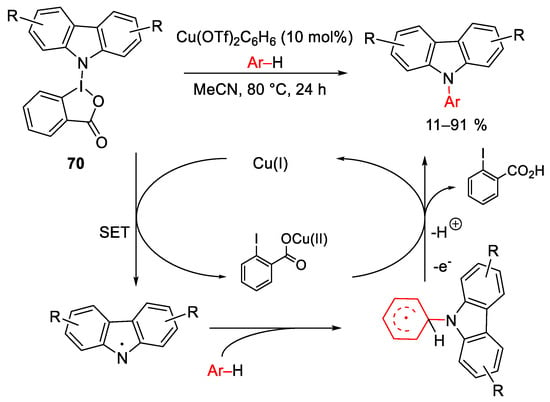
Scheme 93. Copper-catalyzed electrophilic amination of aryl compounds with carbazole-hypervalent iodane(III) reagents.
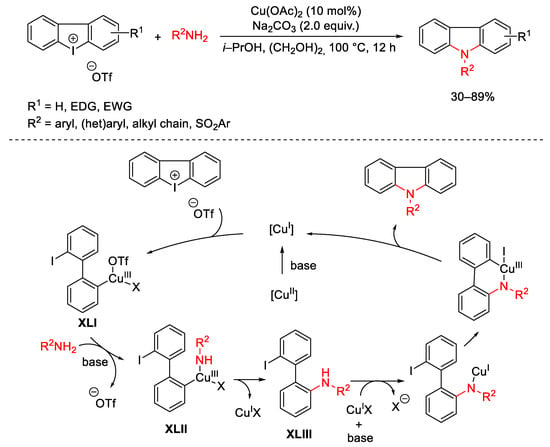
Scheme 94. One-pot copper-catalyzed amine insertion into cyclic diaryliodonium reagents.
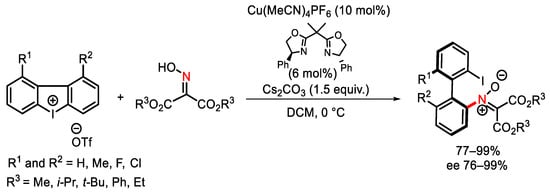
Scheme 95. Copper-catalyzed synthesis of atropisomeric nitrones.
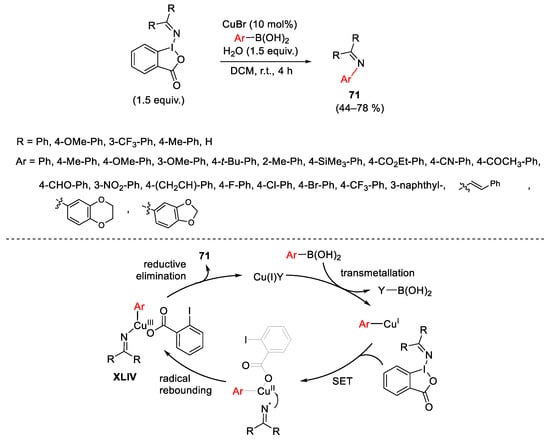
Scheme 96. Copper-catalyzed synthesis of N-aryl(alkenyl) imines using hypervalent iodane (III) reagent containing (diarylmethylene)amino groups.
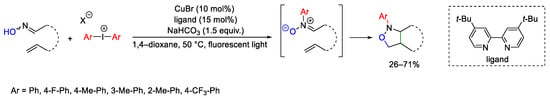
Scheme 97. Copper-catalyzed synthesis of N-aryl-sioxazolidines.
3.2. Azidation Reactions
Organic azides are of great interest in different scientific fields (i.e., chemistry, medicinal chemistry, biology, and material science). This justifies an increase in the development of new methods for their synthesis with different transition metal catalysts [133][134][135] and also with the combination of a copper catalyst with an hypervalent iodine(III) as sources of azide. CuCl was used by Suna to promote an indirect C−H azidation of indoles using unsymmetrical indolyl(phenyl)iodonium azides 72, easily prepared by treating the corresponding indole substrates with a mixture of PhI(OAc)2 and TsOH followed by the addition of sodium azide (Scheme 98) [136]. Then, the subsequent introduction of the copper catalyst to the reaction mixture promoted fragmentation to 3-indolyl azides 73. Finally, these latter were in situ either reduced to heteroarylamines or submitted to Cu-catalyzed cycloaddition reaction to give the correspondent 1,2,3-triazole compounds. The formation of the iodonium azide 72 is consistent with products arising from SEAr reactions. On the other hand, the regioselectivity of the fragmentation of unsymmetrical iodonium salts 72 is controlled by the Cu(I) salt because in catalytic coupling reactions, electron-rich or less sterically hindered aryl moieties are selectively transferred to the metal. Furthermore, the authors indicated the π-Cu(III) complex XLV as the key reaction intermediate, which could either directly evolve to 73 or pass through the heteroarylcopper(III) XLVI due to loss of PhI followed by reductive elimination.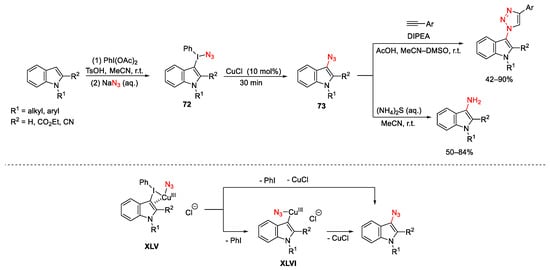
Scheme 98. Copper-catalyzed azidation of indoles.
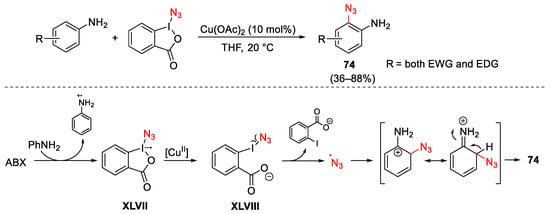
Scheme 99. Direct C–H azidation of anilines with ABX under copper catalysis.
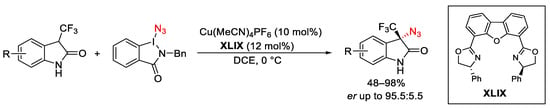
Scheme 100. Direct C–H azidation of 3-trifluoromethyl oxindoles with ABX under copper catalysis.
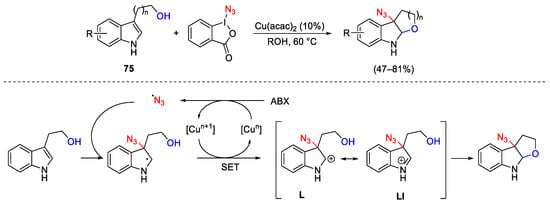
Scheme 101. Intramolecular copper-catalyzed alkoxyazidation of tryptophols.
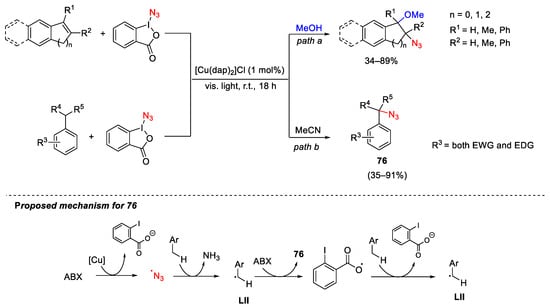
Scheme 102. Azidation of styrene-type double bond (path a) and benzylic C-H bonds (path b) with ABX under copper-photoredox catalysis.
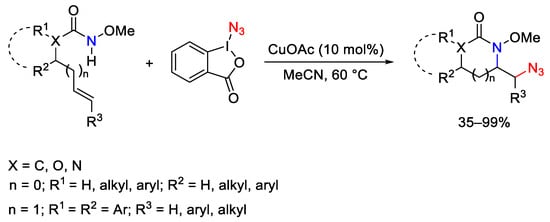
Scheme 103. Intramolecular copper-catalyzed aminoazidation of unactivated alkenes with ABX.
4. Hypervalent Iodine as Reagent in C-O Bond Formation
Moving the attention toward oxygen nucleophiles, the versatility of unsymmetrical diaryliodonium reagents was exploited again by Suma for the C-H oxyarylation of electron-rich heteroarenes or arenes (Scheme 104) [143]. In this context, the same copper-based catalytic system and the same reactivity of unsymmetrical diaryliodonium reagents previously reported in SchemeScheme 85 85 for the synthesis of aryl- and heteroaryl- aniline derivatives, [104] were extended also to build diaryl or heteroaryl ether compounds. As usual, diaryliodonium salts bearing one bulky mesityl phenyl residue allow the selective transfer of the less hindered aryl ring. Furthermore, the regioselectivity of the C−H oxyarylation is controlled at the stage of the formation of iodane 77. Thus, monosubstituted phenyl compounds underwent para C−H iodination and then oxyarylation, while the C-I bond formation in poli-substituted arenes proceeded at the para-position to the strongest electron-donating group.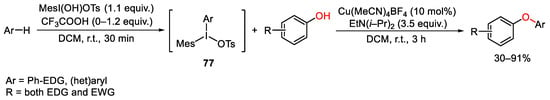
Scheme 104. Copper(I)-catalyzed C-H oxyarylation of phenol derivatives.
Scheme 105. Copper(II)-catalyzed arylation of carboxylates.
Scheme 106. Copper(II)-catalyzed synthesis acyloxylated 2-iodobiaryl.
Scheme 107. Copper(I)-catalyzed C-H oxyarylation of phosphonates with diaryliodonium triflates reagents.
Scheme 108. CuI-catalyzed synthesis of esters of 2′-iodosubstituted biarylphosphinic acids.
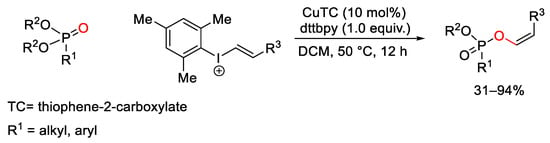
Scheme 109. Copper(I)-catalyzed C-H oxyalkenylation of phosphonates with hypervalent iodine reagents.
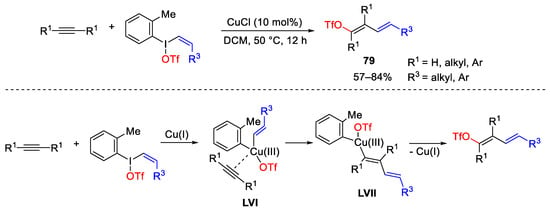
Scheme 110. Copper-catalyzed synthesis of alkenyl triflates using vinyl(aryl)iodonium triflate reagents.
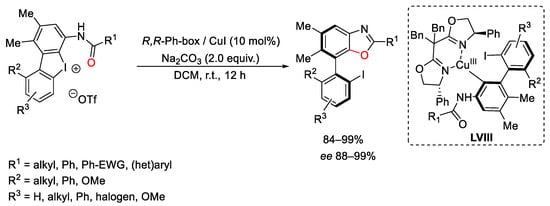
Scheme 111. Copper-catalyzed benzoxazoles formation with cyclic diaryliodonium compounds.
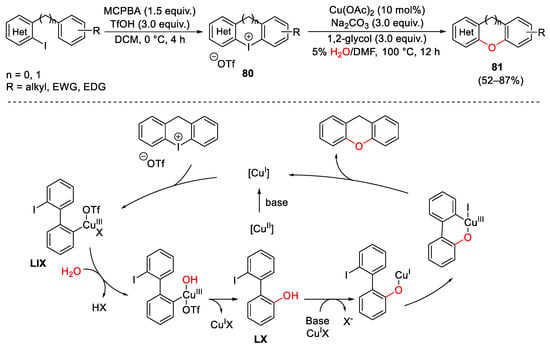
Scheme 112. Copper-catalyzed water insertion into cyclic diaryl iodonium reagents.
Scheme 113. Copper-catalyzed intramolecular aryl-etherification of alkoxyl alkynes.
Scheme 114. Possible mechanism for the intramolecular aryl-etherification of alkoxyl alkynes.
5. Hypervalent Iodine as Reagent in C-S Bond Formation
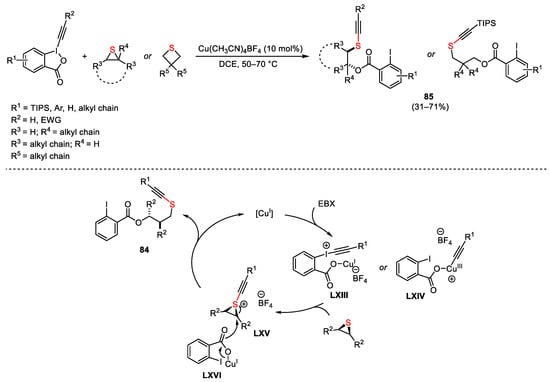
Diaryl tricyclic iodonium salts with general formula 84 could react with various sulphur nucleophiles allowing access to a broad range of poly-heterocyclic or biaryl frameworks [157][158][159][160]. Furthermore, a remarkable Cu-catalyzed enantioselective synthesis of diarylmethanes in the presence of bis(oxazoline) chiral ligands LXII using thio-carboxylate nucleophiles was described by Gu in 2018 (Scheme 115) [161]. The authors attributed the stereochemical course of the process to steric effect associated with both the shape of 84 and the geometry of the Cu(I)/L complex. In particular, the Cu(I)/L complex underwent oxidative addition from the less sterically hindered side of 84. The next coordination of the nucleophile to the Cu(III) centre followed by reductive elimination, gave the final products.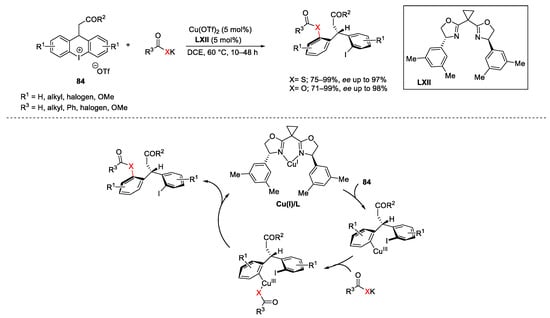
Scheme 115. Cu-catalyzed enantioselective diarylmethanes formation.
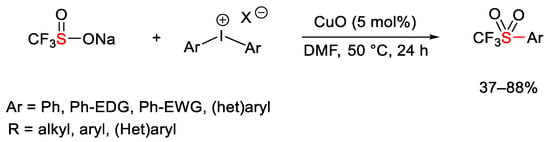
Scheme 116. Copper-catalyzed synthesis of aryltrifluoromethylsulfones.
Scheme 117. Copper-catalyzed ring opening of sulphur heterocycles using EBX reagent.
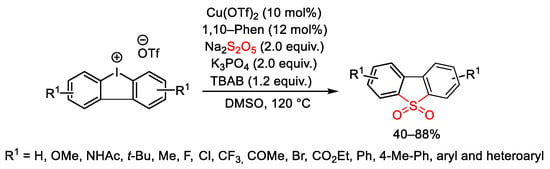
Scheme 118. Copper-catalyzed synthesis of diarylannulated sulfones through SO2/I exchange of cyclic diaryliodonium salts.
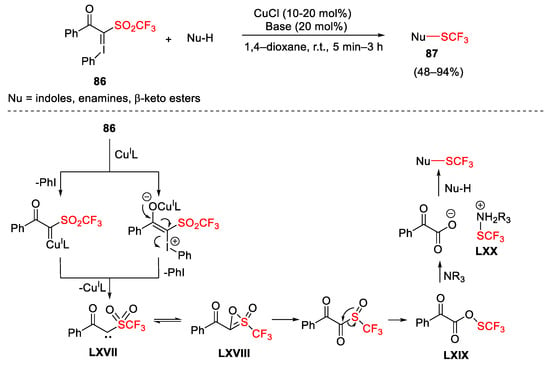
Scheme 119. Copper-catalyzed trifluoromethyl thiolation of nucleophiles with hypervalent iodonium ylide.
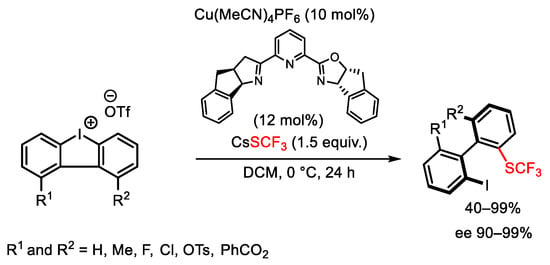
Scheme 120. Atroposelective synthesis of trifluoromethylthiolated biaryls.
Scheme 121. Synthesis of quinoline derivatives.
6. Hypervalent Iodine as Reagent in C-X Bond Formation
The versatility of hypervalent iodine reagents was exploited for the halo-functionalization of a range of sp2 C-H bonds. However—although halogenated compounds are widely studied and synthesized with copper catalysts [170][171][172]—the use of iodines(III) in combination with copper catalysts is rather limited. The fluoro-iodine analogue of the Togni reagent I (Scheme 122) was used for carbo-fluorination of terminal alkenes under copper catalysis by Szabò research group [173]. In particular, [Cu(MeCN)4]BF4 proved to be the best catalyst for intramolecular carbo-cyclization/fluorination of alkenyl malonate compounds.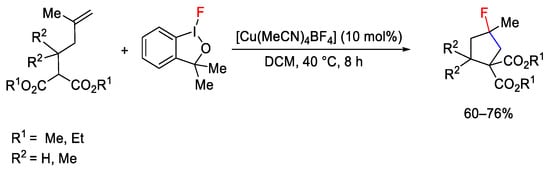
Scheme 122. Hypervalent iodine as reagent in fluorocyclization.
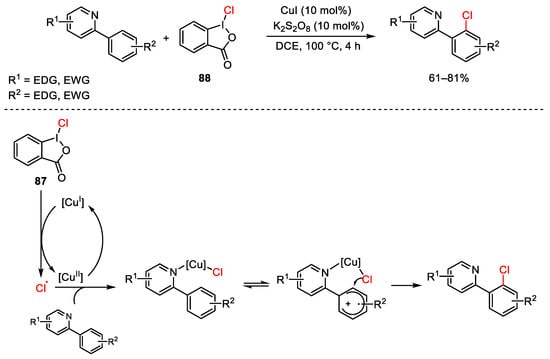
Scheme 123. Regioselective chlorination of C-H aromatic bonds.
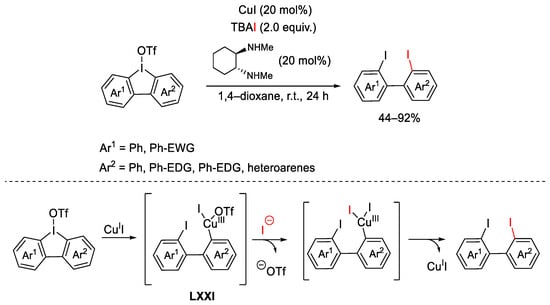
Scheme 124. Iodinative ring opening of cyclic hypervalent iodine reagents.
7. Hypervalent Iodine as Reagent in C-P Bond Formation
Procedures for copper-mediated coupling of phosphorus nucleophiles with diaryliodonium reagents were also disclosed. In 2013, Zhao reported a copper(I)chloride P-arylation process of achiral diaryl phosphine oxides and H-phosphonates nucleophile with symmetrical and unsymmetrical diaryliodonium salts (Scheme 125) [177]. The reaction proceeded at room temperature, in short reaction time and with a broad reaction scope. Furthermore, when unsymmetrical iodonium salts were used, reaction occurred preferentially on the sterically hindered—or the more electron-deficient—aromatic ring.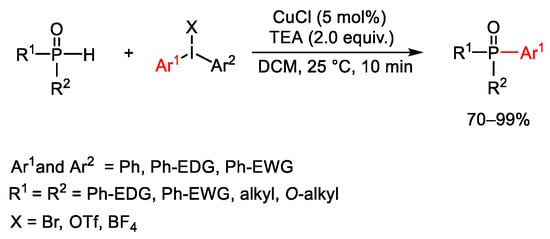
Scheme 125. Copper catalyzed P-Arylation using diaryliodonium reagents.
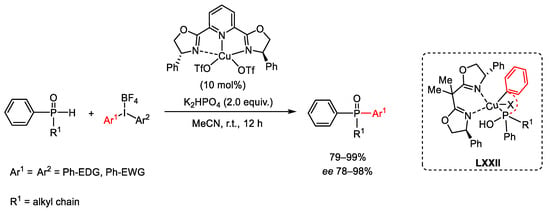
Scheme 126. Enantioselective copper-catalyzed P-Arylation using diaryliodonium reagents.
8. Hypervalent Iodines as Oxidant
The use of hypervalent iodine as oxidant to provide coupling reactions involving the formation of different C-C and C-N bonds is an elegant and efficient synthetic pathway to afford heterocyclic systems. In this context, the use of N-sulfonyl 2-amidobiphenyls 89 as substrates allowed carbazoles 90 to be obtained, under both Cu-catalyzed and metal-free conditions, with generally lower yields in the latter case [179]. The observed kinetic insensitivity to the copper catalyst led to propose that the copper species worked as a Lewis acid to activate hypervalent iodine(III) oxidant. Moreover, when the radical scavenger was added to the reaction mixture, the reaction was significantly decreased, suggesting that a radical intermediate was involved. The reaction realized in the presence of Cu(OTf)2 in DCE at 50 °C was compatible with electron-donating substituents at the nucleophilic phenyl part (right side) and the electron-withdrawing groups on the “left” rings facilitated the cyclization (Scheme 127).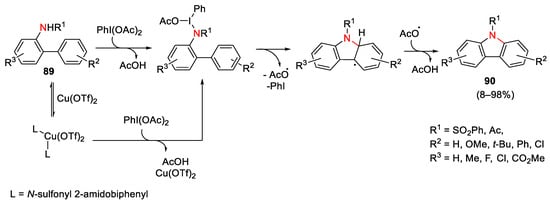
Scheme 127. Mechanism for the synthesis of carbazole under Cu-catalyzed or metal-free conditions using hypervalent iodine (III) as oxidant.
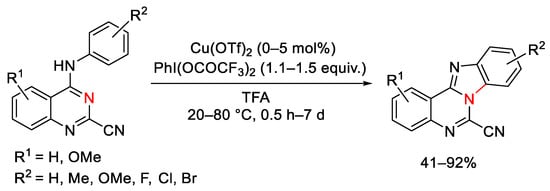
Scheme 128. Copper(II)-mediated oxidative ring closure of 4-anilinoquinazoline-2-carbonitriles.
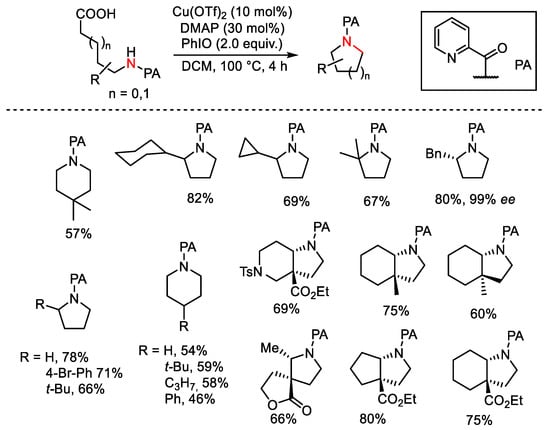
Scheme 129. Copper-catalyzed intramolecular decarboxylative C-N coupling of aliphatic carboxylic acids.
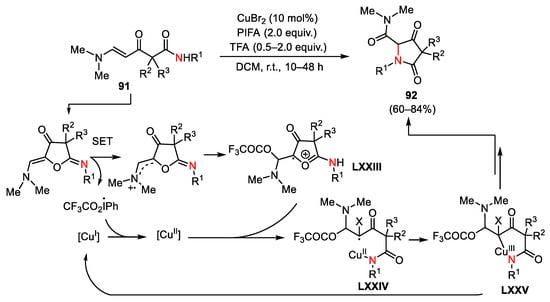
Scheme 130. Plausible mechanism for the formation of pyrrolidine-2,4-diones.
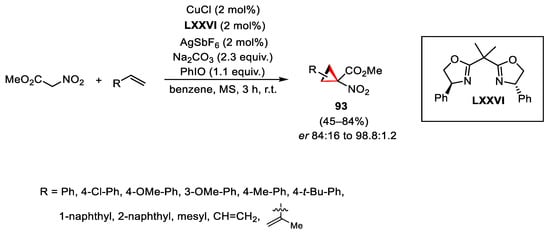
Scheme 131. Copper-catalyzed cyclopropanation reaction.
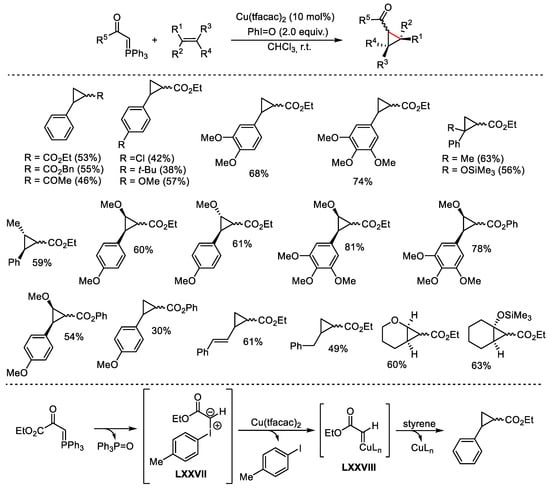
Scheme 132. Cyclopropanation of alkenes in the presence of catalytic Cu(tfacac)2 and iodosotoluene.
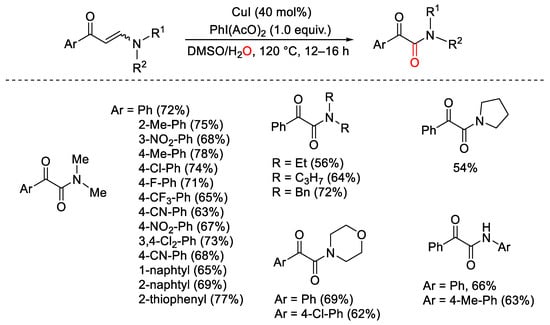
Scheme 133. Synthesis of α-keto amides from enaminones under the copper/PhI(OAc)2 catalytic system.
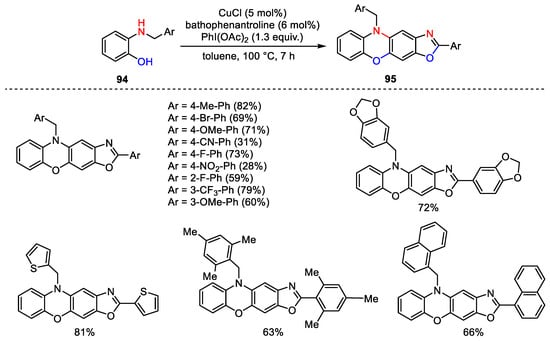
Scheme 134. Dimerization/cyclization of 2-benzylaminophenols.
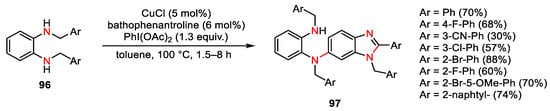
Scheme 135. Dimerization/cyclization of N,N′-dibenzyl-1,2-benzenediamines.
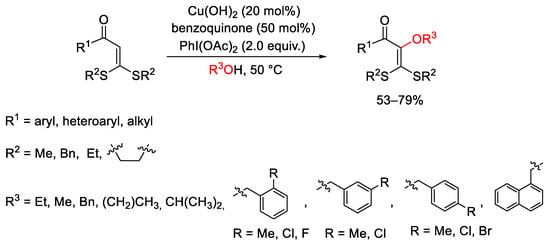
Scheme 136. Alkoxylation of S,S-functionalized internal olefins.
References
- Zhdankin, V.V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds; Wiley: Chichester, UK, 2013.
- Richardson, R.D.; Wirth, T. Hypervalent Iodine Goes Catalytic. Angew. Chem. Int. Ed. 2006, 45, 4402–4404.
- Wirth, T. Hypervalent Iodine Chemistry in Synthesis: Scope and New Directions. Angew. Chem. Int. Ed. 2005, 44, 3656–3665.
- Wirth, T. Hypervalent Iodine Chemistry; Springer: Berlin/Heidelberg, Germany, 2003.
- Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press: San Diego, CA, USA, 1996.
- Le Du, E.; Waser, J. Recent progress in alkynylation with hypervalent iodine reagents. Chem. Commun. 2023, 59, 1589–1604.
- Simonet-Davin, R.; Waser, J. Azidation with Hypervalent Iodine Reagents. Synthesis 2023, 55, 1652–1661.
- Yoshimura, A.; Zhdanlin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435.
- Shetgaonkar, S.E.; Singh, F.V. Catalytic stereoselective synthesis involving hypervalent iodine-based chiral auxiliaries. Org. Biomol. Chem. 2023, 21, 4163–4180.
- Singh, F.V.; Shetgaonkar, S.E. Progress in organocatalysis with hypervalent iodine cataysts. Chem. Soc. Rev. 2022, 51, 8102–8139.
- Rani, N.; Soni, R.; Sihag, M.; Kinger, M.; Aneja, D.K. Combined Approach of Hypervalent Iodine Reagents and Transition Metals in Organic Reactions. Adv. Synth. Catal. 2022, 364, 1798–1848.
- Shetgaonkar, S.E.; Mamgain, R.; Kikushima, K.; Dohi, T.; Singh, F.V. Palladium-Catalyzed Organic Reactions Involving Hypervalent Iodine Reagents. Molecules 2022, 27, 3900.
- Sun, T.-Y.; Wang, X.; Geng, H.; Xie, Y.; Wu, Y.-D.; Zhang, X.; Schaefer, H.F. III Why does Togni’s Reagent I exist in the High-Energy Hypervalent Iodine Form? Re-Evaluation of Benziodoxole Based Hypervalent Iodine Reagents. Chem. Commun. 2016, 52, 5371–5374.
- Zhdankin, V.V. Benziodoxole-Based Hypervalent Iodine Reagents in Organic Synthesis. Curr. Org. Synth. 2005, 2, 121–145.
- Nicolaou, K.C.; Mathison, C.J.N.; Montagnon, T. o-Iodoxybenzoic Acid (IBX) as a Viable Reagent in the Manipulation of Ni-trogen- and Sulfur-Containing Substrates: Scope, Generality, and Mechanism of IBX-Mediated Amine Oxidations and Dithiane Deprotections. J. Am. Chem. Soc. 2004, 126, 5192–5201.
- Nicolaou, K.C.; Baran, P.S.; Zhong, Y.-L.; Barluenga, S.; Hunt, K.W.; Kranich, R.; Vega, J.A. Iodine(V) Reagents in Organic Synthesis. Part 3. New Routes to Heterocyclic Compounds via o-Iodoxybenzoic Acid-Mediated Cyclizations: Generality, Scope, and Mechanism. J. Am. Chem. Soc. 2002, 124, 2233–2244.
- Zhdankin, V.V.; Stang, P.J. Chemistry of Polyvalent Iodine. Chem. Rev. 2008, 108, 5299–5358.
- Meyer, S.D.; Schreiber, S.L. Accelerationof the Dess-Martin oxidation by water. J. Org. Chem. 1994, 59, 7549–7552.
- Duschek, A.; Kirh, S.F. 2-Iodoxybenzoic Acid—A Simple Oxidant with a Dazzling Array of Potential Applications. Angew. Chem. Int. Ed. 2011, 50, 1524–1552.
- Wirth, T. IBX—New Reactions with an Old Reagent. Angew. Chem. Int. Ed. 2001, 40, 2812–2814.
- Ochiai, M.; Sueda, T.; Miyamoto, K.; Kriprof, P.; Zhdankin, V.V. Trans Influences on Hypervalent Bonding of Aryl λ3-Iodanes: Their Stabilities and Isodesmic Reactions of Benziodoxolones and Benziodazolones. Angew. Chem. Int. Ed. 2006, 45, 8203–8206.
- Zhdankin, V.V.; Kuehl, C.J.; Krasunsky, A.P.; Bolz, J.T.; Simonsen, A.J. 1-(Organosulfonyloxy)-3(1H)-1,2-benziodoxoles: Preparation and Reactions with Alkynyltrimethylsilanes. J. Org. Chem. 1996, 61, 6547–6551.
- Ochiai, M.; Masaki, Y.; Shiro, M. Synthesis and Structure of 1-Alkynyl-1,2-Benziodoxol-3(1H)-Ones. J. Org. Chem. 1991, 56, 5511–5513.
- Zhdankin, V.V.; Krasutsky, A.P.; Kuehl, C.J.; Simonsen, A.J.; Woodward, J.K.; Mismash, B.; Bolz, J.T. Preparation, X-Ray Crystal Structure, and Chemistry of Stable Azidoiodinanes Derivatives of Benziodoxole. J. Am. Chem. Soc. 1996, 118, 5192–5197.
- Zhdankin, V.V.; Kuehl, C.J.; Krasutsky, A.P.; Bolz, J.T.; Mismash, B.; Woodward, J.K.; Simonsen, A.J. 1-Cyano-3-(1H)-1,2-Benziodoxols: Stable Cyanoiodinanes and Efficient Reagents for Direct N-Alkyl Cyanation of N,N-Dialkylarylamines. Tetrahedron Lett. 1995, 36, 7975–7978.
- Daugulis, O.; Do, H.-Q.; Shabashov, D. Palladium- and Copper-Catalyzed Arylation of Carbon-Hydrogen Bonds. Acc. Chem. Res. 2009, 42, 1074–1086.
- Fañanás-Mastral, M. Copper-Catalyzed Arylation with Diaryliodonium Salts. Synthesis 2017, 49, 1905–1930.
- Loro, C.; Oble, J.; Foschi, F.; Papis, M.; Beccalli, E.M.; Giofrè, S.; Poli, G.; Broggini, G. Acid-mediated decarboxylatiove C-H coupling between arenes and O-allyl carbamates. Org. Chem. Front. 2022, 9, 1711–1718.
- Phipps, R.J.; Gaunt, M.J. A Meta-Selective Copper-Catalyzed C-H Bond Arylation. Science 2009, 323, 1593–1597.
- Chen, B.; Hou, X.-L.; Li, Y.-X.; Wu, Y.-D. Mechanistic Understanding of the Unespected Meta Selectivity in Copper-Catalyzed Anilide C-H Bond Arylation. J. Am. Chem. Soc. 2011, 133, 7668–7671.
- Duong, H.A.; Gilligan, R.E.; Cooke, M.L.; Phipps, R.J.; Gaunt, M.J. Copper(II)-Catalyzed meta-Selective Direct Arylation of α-Aryl Carbonyl Compounds. Angew. Chem. Int. Ed. 2011, 50, 463–466.
- Ciana, C.-L.; Phipps, R.J.; Brandt, J.R.; Meyer, F.-M.; Gaunt, M.J. A Highly Para-Selective Copper(II)-Catalyzed Direct Arylation of Aniline and Phenol Derivatives. Angew. Chem. Int. Ed. 2011, 50, 458–462.
- Phipps, R.J.; Grimster, R.P.; Gaunt, M.J. Cu(II)-Catalyzed Direct and Site-Selective Arylation of Indoles Under Mild Conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174.
- Liang, H.; Zhu, G.; Pu, X.; Qiu, L. Copper-Catalyzed Enantioselective C-H Arylation between 2-Arylindoles and Hypervalent Iodine Reagents. Org. Lett. 2021, 23, 9246–9250.
- Pitts, A.K.; O’Hara, F.; Snell, R.H.; Gaunt, M.J. A Concise and Scalable Strategy for the Total Synthesis of Dictyodendrin B Based on Sequential C-H- Functionalization. Angew. Chem. Int. Ed. 2015, 54, 5451–5455.
- Yang, Y.; Gao, P.; Zhao, Y.; Shi, Z. Regiocontrolled Direct C-H Arylation of Indoles at the C4 and C5 Positions. Angew. Chem. Int. Ed. 2017, 56, 3966–3971.
- Yang, Y.; Li, R.; Zhao, Y.; Zhao, D.; Shi, Z. Cu-Catalyzed Direct C6-Arylation of Indoles. J. Am. Chem. Soc. 2016, 138, 8734–8737.
- Modha, S.G.; Greaney, M.F. Atom-Economical Transformation od Diaryliodoniu, Salts: Tandem C-H and N-H Arylation of Indoles. J. Am. Chem. Soc. 2015, 137, 1416–1419.
- Allen, A.E.; MacMillan, D.W.C. Enantioselective α-Arylation of Aldehydes via the Productive Merger of Iodonium Salts and Organocatalysis. J. Am. Chem. Soc. 2011, 133, 4260–4263.
- Pan, J.-L.; Chen, T.; Zhang, Z.-Q.; Li, Y.-F.; Zhang, X.-M.; Zhang, F.-M. A Cu-mediated one-pot Michael addition/α-arylation strategy using a diaryliodonium salt: A direct and efficient approach to α-aryl-β-substituted cyclic ketone scaffolds. Chem. Commun. 2016, 52, 2382–2385.
- Bigot, A.; Williamson, A.E.; Gaunt, M.J. Enantioselective α-Arylation of N-Acyloxazolidinones with Copper(II)-bisoxazoline Catalysts and Diaryliodonium Salts. J. Am. Chem. Soc. 2011, 133, 13778–13781.
- Harvey, J.S.; Simonovich, S.P.; Jamison, C.R.; MacMillan, D.W.C. Enantioselective α-Arylation od Carbonyls via Cu(I)-Bisoxazoline Catalysis. J. Am. Chem. Soc. 2011, 133, 13782–13785.
- Zhu, S.; MacMillan, D.W.C. Enantioselective Copper-Catalyzed Construction of Aryl Pyrrolindolines via an Aryla-tion-Cyclization Cascade. J. Am. Chem. Soc. 2012, 134, 10815–10818.
- Kieffer, M.E.; Chuang, K.V.; Reisman, S.E. A copper-catalyzed arylation of tryptamines for the direct synthesis of aryl pyr-roloindolines. Chem. Sci. 2012, 3, 3170–3174.
- Kieffer, M.E.; Chuangm, K.V.; Reinsman, S.E. Copper-Catalyzed Diasteroselective Arylation of Tryptophan Derivatives: Total Synthesis of (+)-Naseseazines A and B. J. Am. Chem. Soc. 2013, 135, 5557–5560.
- Phipps, R.J.; McMurray, L.; Ritter, S.; Doung, H.A.; Gaunt, M.J. Copper-Catalyzed Alkene Arylation with Diaryliodonium Salts. J. Am. Chem. Soc. 2012, 134, 10773–10776.
- Almasalma, A.A.; Mejia, E. 1-Phenyl-1,2-benziodoxol-3-(1H)-one as Synthon for Phthalide Synthesis through Pd-Free, Base-Free, Sonoganshira-Type Couple Cyclization Reaction. Eur. J. Org. Chem. 2018, 2018, 188–195.
- Xu, Z.-F.; Cai, C.-X.; Liu, J.-T. Copper-Catalyzed Regioselective Reaction of Internal Alkenes and Diaryliodonium Salts. Org. Lett. 2013, 15, 2096–2099.
- Walkinshaw, A.J.; Xu, W.; Suero, M.G.; Gaunt, M.J. Copper-Catalyzed Carboarylation of Alkynes via Vinyl Cations. J. Am. Chem. Soc. 2013, 135, 12532–12535.
- Peng, J.; Chen, C.; Chen, J.; Su, X.; Xi, C.; Chen, H. Cu-Catalyzed Arylcarbocyclization of Alkynes with Diaryliodonium Salts through C-C Bond Formation on Inert C(sp3)-H Bond. Org. Lett. 2014, 16, 3776–3779.
- Zhang, F.; Das, S.; Walkingshaw, A.J.; Casitas, A.; Taylor, M.; Suero, M.G.; Gaunt, M.J. Cu-Catalyzed Cascades to Carbocyles: Union of Diaryliodonium Salts with Alkenes or Alkynes Exploiting Remote Carbocations. J. Am. Chem. Soc. 2014, 136, 8851–8854.
- Chen, J.; Chen, C.; Chen, J.; Wang, G.; Qu, H. Cu-catalyzed intramolecular aryl-etherification reactions of alkoxyl alkynes with diaryliodonium salts via cleavage of a stable C–O bond. Chem. Commun. 2015, 51, 1356–1359.
- Sinai, A.; Vangel, D.; Gati, T.; Bombincz, P.; Novak, Z. Utilization of Copper-Catalyzed Carboarylation-Ring Closure for the Synthesis of New Oxazoline Derivatives. Org. Lett. 2015, 17, 4136–4139.
- Sinai, A.; Meszaros, A.; Gati, T.; Kudar, V.; Pallo, A.; Novak, Z. Copper-Catalyzed Oxidative Ring Closure and Carboarylation of 2-Ethynylanilides. Org. Lett. 2013, 15, 5654–5657.
- Collins, B.S.L.; Suero, M.G.; Gaunt, M.J. Copper-Catalyzed Arylative Meyer-Schuster Rearrangement of Propargylic Alcohols to Complex Enones Using Diaryliodonium Salts. Angew. Chem. Int. Ed. 2013, 52, 5799–5802.
- Cahard, E.; Bremeyer, N.; Gaunt, M.J. Copper-Catalyzed Intramolecular Electrophilic Carbofunctionalization of Allylic Amides. Angew. Chem. Int. Ed. 2013, 52, 9284–9288.
- Cahard, E.; Male, H.P.J.; Tissot, M.; Gaunt, M.J. Enantioselective and Regiodivergent Copper-Catalyzed Electrophilic Arylation of Allylic Amides with Diaryliodonium Salts. J. Am. Chem. Soc. 2015, 137, 7986–7989.
- Gigant, N.; Chausset-Boissarie, L.; Belhomme, M.-C.; Poisson, T.; Pannecoucke, X.; Gillaizeau, I. Copper-Catalyzed Direct Ar-ylation of Cyclic Enamides Using Diaryliodonium Salts. Org. Lett. 2013, 15, 278–281.
- Prakash, M.; Muthusamy, S.; Kesavan, V. Copper(I) Bromide Catalyzed Arylation of Cyclic Enamides and Naph-thyl-1-acetamides Using Diaryliodonium Salts. J. Org. Chem. 2014, 79, 7836–7843.
- Kumar, D.; Pilania, M.; Arun, V.; Pooniya, S. C-H arylation of azaheterocycles: A direct ligand-free and Cu-catalyzed approach using diaryliodonium salts. Org. Biomol. Chem. 2014, 12, 6340–6344.
- Hari, D.P.; Waser, J. Copper-Catalyzed Oxy-Alkynylation of Diazo Compounds with Hypervalent Iodine Reagents. J. Am. Chem. Soc. 2016, 138, 2190–2193.
- Hari, D.P.; Schouwey, L.; Baber, V.; Scopelliti, R.; Fadaei-Tirani, F.; Waser, J. Ethynylbenziodazolones (EBZ) as Electrophilic Alkynylation Reagents for the Highly Enantioselective Copper-Catalyzed Oxyalkynylation of Diazo Compounds. Chem. Eur. J. 2019, 25, 9522–9528.
- Pisella, G.; Gagnebin, A.; Waser, J. Three-Component Reaction for the Synthesis of Highly Functionalized Propargyl Ethers. Chem. Eur. J. 2020, 26, 10199–10204.
- Shen, K.; Wang, Q. Copper-catalyzed aminoalkynylation of alkenes with hypervalent iodine reagents. Chem. Sci. 2017, 8, 8265–8270.
- Teodoro, B.V.M.; Silva, L.F.J. Sequential Michael Addition/Electrophilic Alkynylation: Synthesis of α-Alkynyl-β-Substituted Ketones and Chromanones. J. Org. Chem. 2018, 83, 13604–13611.
- Sun, X.; Guo, X.-Q.; Chen, L.-M.; Kang, T.-R. Synthesis, Characterization of Spirocyclic λ3-Iodanes and Their Application to Prepare 4,1-Benzoxazepine-2,5-diones and 1,3-Diynes. Chem. Eur. J. 2021, 27, 4312–4316.
- Wang, X.; Ye, Y.; Zhang, S.; Feng, J.; Xu, Y.; Zhang, Y.; Wang, J. Copper-Catalyzed C(sp3)-C(sp3) Bond Formation Using a Hypervalent Iodine Reagent: An Efficient Allylic Trifluoromethylation. J. Am. Chem. Soc. 2011, 133, 16410–16413.
- Parsons, A.T.; Buchwald, S.L. Copper-Catalyzed Trifluoromethylation of Unactivated Olefins. Angew. Chem. Int. Ed. 2011, 50, 9120–9123.
- Lonca, G.H.; Ong, D.Y.; Tran, T.M.H.; Tejo, C.; Chiba, S.; Gagosz, F. Anti-Markovinkov Hydrofunctionalization of Alkenes: Usa of a Benzyl Group as a Traceless Redox-Active Hydrogen Donor. Angew. Chem. Int. Ed. 2017, 56, 11440–11444.
- Shimizu, R.; Egami, H.; Hamashima, Y.; Sodeoka, M. Copper-Catalyzed Trifluoromethylation of Allylsilanes. Angew. Chem. Int. Ed. 2012, 51, 4577–4580.
- Wang, X.-P.; Lin, J.-H.; Zhang, C.-P.; Xiao, J.-C.; Zheng, X. Copper-catalyzed trifluoromethylation of alkenes with an electrophilic trifluoromethylating reagent. Beilstein J. Org. Chem. 2013, 9, 2635–2640.
- Weng, Z.; Li, H.; He, W.; Yao, L.-F.; Tan, J.; Chen, J.; Yuan, Y.; Huan, K.-W. Mild copper-catalyzed trifluoromethylation of terminal alkynes using an electrophilic trifluoromethylating reagent. Tetrahedron 2012, 68, 2527–2531.
- Feng, C.; Loh, T.-P. Copper-catalyzed olefinic trifluoromethylation of enamides at room temperature. Chem. Sci. 2012, 3, 3458–3462.
- Feng, C.; Loh, T.-P. Directing-Group-Assisted Copper-Catalyzed Olefinic Trifluoromethylation of Electron-Deficient Alkenes. Angew. Chem. Int. Ed. 2013, 52, 12414–12417.
- Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Catalytic C-H α-Trifluoromethylation of α,β-Unsaturated Carbonyl Compounds. Org. Lett. 2014, 16, 1522–1525.
- Wang, X.; Ye, Y.; Ji, G.; Xu, Y.; Zhang, S.; Feng, J.; Zhang, Y.; Wang, J. Copper-Catalyzed Direct C-H Trifluoromethylation of Quinones. Org. Lett. 2013, 15, 3730–3733.
- Deng, Q.-H.; Wadepohl, H.; Gade, L.H. Highly Enantioselective Copper-Catalyzed Electrophilic Trifluoromethylation of b-Ketoesters. J. Am. Chem. Soc. 2012, 134, 10769–10772.
- Allen, A.E.; MacMillan, D.W.C. The Productive Merger of Iodonium Salts and Organocatalysis: A Non-photolytic Approach to the Enantioselective α-Trifluoromethylation of Aldehydes. J. Am. Chem. Soc. 2010, 132, 4986–4987.
- Pair, E.; Monteiro, N.; Bouyssi, D.; Baudoin, O. Copper-Catalyzed Trifluoromethylation of N,N-Dialkylhydrazones. Angew. Chem. Int. Ed. 2013, 52, 5346–5349.
- Prieto, A.; Jearmet, E.; Monteiro, N.; Bouyssi, D.; Baudoin, O. Copper-Catalyzed b-Trifluoromethylation of Conjugated Hydrazones. Org. Lett. 2014, 16, 4770–4773.
- Prieto, A.; Bouyssi, D.; Monteiro, N. Copper-Catalyzed Trifluoromethylation od Hydrazones Leading to the Formation of Quaternary α-Trifluoromethyl Diazenes. Asian J. Org. Chem. 2016, 5, 742–745.
- Wu, S.; Li, J.; He, R.; Jia, K.; Chen, Y. Terminal Trifluoromethylation of Ketones via Selective C-C Cleavage of Cycloalkanols Enabled by Hypervalent Iodine Reagents. Org. Lett. 2021, 23, 9204–9209.
- Xiong, Y.-P.; Wu, M.-Y.; Zhang, X.-Y.; Ma, C.-L.; Huang, L.; Zhao, L.-J.; Tan, B.; Liu, X.-Y. Direct Access to α-Trifluoromethyl Enones via Efficient Copper-Catalyzed Trifluoromethylation of Meyer-Schuster Rearrangement. Org. Lett. 2014, 16, 1000–1003.
- Cai, S.; Chen, C.; Sun, Z.; Xi, C. CuCl-catalyzed ortho trifluoromethylation of arenes and heteroarenes with a pivalamido directing group. Chem. Commun. 2013, 49, 4552–4554.
- Liu, T.; Shen, Q. Copper-Catalyzed Trifluoromethylation of Aryl and Vinyl Boronic Acids with An Electrophilic Trifluoro-methylating Reagent. Org. Lett. 2011, 13, 2342–2345.
- Huang, Y.; Fang, X.; Lin, X.; Li, H.; He, W.; Huang, K.-W.; Yuan, Y.; Weng, Z. Room-temperature base-free copper-catalyzed trifluoromethylation of organotrifluoroborates to trifluoromethylarenes. Tetrahedron 2012, 68, 9949–9953.
- Zheng, H.; Huang, Y.; Wang, Z.; Li, H.; Huang, K.-W.; Yuan, Y.; Weng, Z. Synthesis of trifluoromethylated acetylenes via copper-catalyzed trifluoromethylation of alkynyltrifluoroborates. Tetrahedron Lett. 2012, 53, 6646–6649.
- Shimizu, R.; Egami, H.; Nagi, T.; Chae, J.; Hamashima, Y.; Sodeoka, M. Direct C2-trifluoromethylation of indole derivatives catalyzed by copper acetate. Tetrahedron Lett. 2010, 51, 5947–5949.
- Janson, P.G.; Ghoneim, I.; Ilchenko, N.O.; Szabó, K.J. Electrophilic Trifluoromethylation by Copper-Catalyzed Addition of CF3-Transfer Reagents to Alkenes and Alkynes. Org. Lett. 2012, 14, 2882–2885.
- Egami, H.; Shimizu, R.; Sodeoka, M. Oxytrifluoromethylation of multiple bonds using copper catalyst under mild conditions. Tetrahedron Lett. 2012, 53, 5503–5506.
- Ramkumar, N.; Baumane, L.; Zacs, D.; Veliks, J. Merging Copper(I) Photoredox Catalysis and Iodine(III) Chemistry for the Oxy-monofluoromethylation of Alkenes. Angew. Chem. Int. Ed. 2023, 62, e202219027.
- Wang, F.; Qi, X.; Liang, Z.; Chen, P.; Liu, G. Copper-Catalyzed Intermolecular Trifluoromethylazidation of Alkenes: Convenient Access to CF3-Containing Alkyl Azides. Angew. Chem. Int. Ed. 2014, 53, 1881–1886.
- Ilchenko, N.O.; Janson, P.G.; Szabo, K.J. Copper-Mediated Cyanotrifluoromethylation of Styrenes Using the Togni Reagent. J. Org. Chem. 2013, 78, 11087–11091.
- Egami, H.; Kawamura, S.; Miyazaki, A.; Sodeaoka, M. Trifluoromethylation Reactions for the Synthesis of β-Trifluoromethylamines. Angew. Chem. Int. Ed. 2013, 125, 7995–7998.
- Kawamura, S.; Egami, H.; Sodeoka, M. Aminotrifluoromethylation of Olefins via Cyclic Amine Formation: Mechanistic Study and Application to Synthesis of Trifluoromethylated Pyrrolidines. J. Am. Chem. Soc. 2015, 137, 4865–4873.
- Shen, K.; Wang, Q. Copper-catalyzed aminotrifluoromethylayion of alkenes: A facile synthesis of CF3-containing lactams. Org. Chem. Front. 2016, 3, 222–226.
- Zhu, R.; Buchwald, S.L. Copper-Catalyzed Oxytrifluoromethylation of Unactivated Alkenes. J. Am. Chem. Soc. 2012, 134, 12462–12465.
- Zhu, R.; Buchwald, S.L. Enantioselective Functionalization of Radical Intermediates in Redox Catalysis: Copper-Catalyzed Asymmetric Oxytrifluoromethylation of Alkenes. Angew. Chem. Int. Ed. 2013, 52, 12655–12658.
- Lu, D.-F.; Zhu, C.-L.; Xu, H. Copper(I)-catalyzed diastereoselective hydroxytrifluoromethylation of dienes accelerated by phosphine ligands. Chem. Sci. 2013, 4, 2478–2482.
- Yu, Q.; Ma, S. Copper-Catalyzed Cyclic Oxytrifluoromethylation of 2,3-Allenoic Acids to Trifluoromethylated Butenolides. Chem. Eur. J. 2013, 19, 13304–13308.
- He, Y.-T.; Li, L.-H.; Yang, Y.-F.; Wang, Y.-Q.; Luo, J.-Y.; Liu, X.-Y.; Liang, Y.-M. Copper-catalyzed synthesis od trifluoromethyl-substituted isoxazolines. Chem. Commun. 2013, 49, 5687–5689.
- Gao, P.; Yan, X.-B.; Tao, T.; Yang, F.; He, T.; Song, Z.-R.; Liu, X.-L.; Liang, Y.-M. Copper-Catalyzed Trifluoromethylation-Cyclization of Enynes: Highly Regioselective Construction of Trifluoromethylated Carbocycles and Heterocycles. Chem. Eur. J. 2013, 19, 14420–14424.
- Egami, H.; Shimizu, R.; Kawamura, S.; Sodeoka, M. Alkene Trifluoromethylation Coupled with C-C Bond Formation: Con-struction of Trifluoromethylated Carbocycles and Heterocycles. Angew. Chem. Int. Ed. 2013, 52, 4000–4003.
- Lin, J.-S.; Xiong, Y.-P.; Ma, C.-L.; Zhao, L.-J.; Tan, B.; Liu, X.-Y. Efficient Copper-Catalyzed Direct Intramolecular Aminotrifluoromethylation of Unactivated Alkenes with Diverse Nitrogen-Based Nucleophiles. Chem. Eur. J. 2014, 20, 1332–1340.
- Zhou, T.; Chen, Z.-C. Hypervalent iodine in synthesis. 77. An efficient method for the synthesis of N-arylindoles by the cop-per-catalyzed N-arylation of indole with diaryliodonium salts. Synth. Commun. 2002, 32, 903–907.
- Zhou, T.; Chen, Z.-C. Hypervalent Iodine in Synthesis 85: An Efficient Method for the Synthesis of N-Arylbenzimidazoles by the Copper- Catalyzed N-Arylation of Benzimidazole with Diaryliodonium Salts. Heteroat. Chem. 2002, 13, 617–619.
- Zhou, T.; Chen, Z.C. Hypervalent iodine in synthesis 87: The synthesis of 2,5-diaryl-2H-tetrazoles. J. Chem. Res. 2004, 2004, 404–405.
- Zhou, T.; Li, T.C.; Chen, Z.-C. Hypervalent Iodine in Synthesis. Part 86. Helv. Chim. Acta 2005, 88, 290–296.
- Saikia, R.A.; Barman, D.; Dutta, A.; Thakur, A.J. N1- and N3-Arylations of Hydantoins Employing Diaryliodonium Salts via Copper(I) Catalysis at Room Temperature. Eur. J. Org. Chem. 2021, 2021, 400–410.
- Geng, X.; Mao, S.; Chen, L.; Yu, J.; Han, J.; Hua, J.; Wang, L. Copper-catalyzed direct N-arylsulfonamides using diaryliodonium salts in water. Tetrahedron Lett. 2014, 55, 3856–3859.
- Vaddula, B.; Leazer, J.; Varma, R.S. Copper-Catalyzed Ultrasound-Expedited N-Arylation of Sulfoximines using Diaryliodonium Salts. Adv. Synth. Catal. 2012, 354, 986–990.
- Watanabe, K.; Moriyama, K. Copper-Catalyzed Indole-Selective C–N Coupling Reaction of Indolyl(2-alkoxy-phenyl)iodonium Imides: Effect of Substituent on Iodoarene as Dummy Ligand. J. Org. Chem. 2018, 83, 14827–14833.
- Watanabe, K.; Moriyama, K. Cu-Catalyzed Oxidative 3-Amination of Indoles via Formation of Indolyl(aryl)iodonium Imides Using o-Substituted (Diacetoxyiodo)arene as a High-Performance Hypervalent Iodine Compound. Molecules 2019, 24, 1147.
- Sokolovs, I.; Lubriks, D.; Suna, E. Copper-Catalyzed Intermolecular C–H Amination of (Hetero)arenes via Transient Unsymmetrical λ3-Iodanes. J. Am. Chem. Soc. 2014, 136, 6920–6928.
- Berzina, B.; Sokolovs, I.; Suna, E. Copper-Catalyzed para-Selective C–H Amination of Electron-Rich Arenes. ACS Catal. 2015, 5, 7008–7014.
- Wu, Y.; Izquierdo, S.; Vidossich, P.; Lledýs, A.; Shafir, A. NH-Heterocyclic Aryliodonium Salts and their Selective Conversion into N1-Aryl-5-iodoimidazoles. Angew. Chem. Int. Ed. 2016, 55, 7152–7156.
- Lv, T.; Wang, Z.; You, J.; Lan, J.; Gao, G. Copper-Catalyzed Direct Aryl Quaternization of N-Substituted Imidazoles to Form Imidazolium Salts. J. Org. Chem. 2013, 78, 5723–5730.
- Reus, C.; Stolar, M.; Vanderkley, J.; Nebauer, J.; Baumgartner, T. A Convenient N-Arylation Route for Electron-Deficient Pyridines: The Case of π-Extended Electrochromic Phosphaviologens. J. Am. Chem. Soc. 2015, 137, 11710–11717.
- Li, P.; Cheng, G.; Zhang, H.; Xu, X.; Gao, J.; Cui, X. Copper-Catalyzed One-Pot Synthesis of Unsymmetrical Arylurea Derivatives via Tandem Reaction of Diaryliodonium Salts with N-Arylcyanamide. J. Org. Chem. 2014, 79, 8156–8162.
- Peng, X.; Sun, Z.; Kuang, P.; Li, L.; Chen, J.; Chemn, J. Copper-Catalyzed Selective Arylation of Nitriles with Cyclic Diaryl Iodonium Salts: Direct Access to Structurally Diversified Diarylmethane Amides with Potential Neuroprotective and Anticancer Activities. Org. Lett. 2020, 22, 5789–5795.
- Aradia, K.; Novak, Z. Copper-Catalyzed Oxidative Ring Closure of ortho-Cyanoanilides with Hypervalent Iodonium Salts: Arylation–Ring Closure Approach to Iminobenzoxazines. Adv. Synth. Catal. 2015, 357, 371–376.
- Wang, Y.; Chen, C.; Peng, J.; Li, J. Copper(II)-Catalyzed Three-Component Cascade Annulation of Diaryliodoniums, Nitriles, and Alkynes: A Regioselective Synthesis of Multiply Substituted Quinolines. Angew. Chem. Int. Ed. 2013, 52, 5323–5327.
- Zhang, Y.; Lu, J.; Lan, T.; Cheng, S.; Liu, W.; Chen, C. Preparation, Characterization, and Reactivity of Aliphatic Amino Iodane(III) Reagents. Eur. J. Org. Chem. 2021, 2021, 436–442.
- Tianlei, L.; Yue, Z.; Wei, L.; Chanjuan, X.; Chao, C. Carbazolation Study of Active Arenes with Carbazole-Containing Hypervalent Iodine(III) Reagents. Chin. J. Org. Chem. 2019, 39, 2166–2174.
- Zhu, D.; Liu, Q.; Luo, B.; Chen, M.; Pi, R.; Huang, P.; Wen, S. Synthesis of Carbazoles via One-Pot Copper-Catalyzed Amine Insertion into Cyclic Diphenyleneiodoniums as a Strategy to Generate a Drug-Like Chemical Library. Adv. Synth. Catal. 2013, 355, 2172–2178.
- Xu, S.; Zhao, K.; Gu, Z. Copper-Catalyzed Asymmetric Ring-Opening of Cyclic Diaryliodonium with Benzylic and Aliphatic Amines. Adv. Synth. Catal. 2018, 360, 3877–3883.
- Zhang, X.; Zhao, K.; Li, N.; Yu, J.; Gong, L.-Z.; Gu, Z. Atroposelective Ring Opening of Cyclic Diaryliodonium Salts with Bulky Anilines Controlled by a Chiral Cobalt(III) Anion. Angew. Chem. Int. Ed. 2020, 59, 19899–19904.
- Li, Q.; Zhang, M.; Zhan, S.; Gu, Z. Copper-Catalyzed Enantioselective Ring-Opening of Cyclic Diaryliodoniums and O-Alkylhydroxylamines. Org. Lett. 2019, 21, 6374–6377.
- Chao, Z.; Ma, M.; Gu, Z. Cu-Catalyzed Site-Selective and Enantioselective Ring Opening of Cyclic Diaryliodoniums with 1,2,3-Triazoles. Org. Lett. 2020, 22, 6441–6446.
- Guo, W.; Gu, J.; Gu, Z. Catalytic Asymmteric Synthesis of Atropisomeric Nitrones: Versatile Intermediate for Axially Chiral Biaryls. Org. Lett. 2020, 22, 7622–7628.
- Hu, Y.; Zheng, S.; Fan, W.; Yuana, W. Copper-Catalysed Electrophilic Amination of Aryl(alkenyl) Boronic Acids with Nitrogen-Containing Hypervalent Iodine (III) Reagent. Adv. Synth. Catal. 2021, 363, 4701–4707.
- Tanaka, K.; Yoshida, M.; Yamamoto, A.; Hashimoto, Y.; Morita, N.; Tamura, O. Synthesis of N-Aryl Isoxazolidines by Photo-Promoted N-Selective Arylation of Oximes and Cyclization Using Hypervalent Iodine Reagents and Copper Catalyst. Adv. Synth. Catal. 2023, 365, 1419–1424.
- Sivaguru, P.; Ning, Y.; Bi, X. New Strategies for the Synthesis of Aliphatic Azides. Chem. Rev. 2021, 121, 4253–4307.
- Sala, R.; Loro, C.; Foschi, F.; Broggini, G. Transition Metal Catalyzed Azidation Reactions. Catalysts 2020, 10, 1173.
- Foschi, F.; Loro, C.; Sala, R.; Oble, J.; Lo Presti, L.; Beccalli, E.M.; Poli, G.; Broggini, G. Intramolecular Aminoazidation of Unactivated Terminal Alkenes by Palladium-Catalyzed Reactions with Hydrogen Peroxide as the Oxidant. Org. Lett. 2020, 22, 1402–1406.
- Lubriks, D.; Sokolovs, I.; Suna, E. Indirect C–H Azidation of Heterocycles via Copper-Catalyzed Regioselective Fragmentation of Unsymmetrical λ3-Iodanes. J. Am. Chem. Soc. 2012, 134, 15436–15442.
- Fan, Y.; Wan, W.; Ma, G.; Gao, W.; Jiang, H.; Zhuc, S.; Hao, J. Room-Temperature Cu(ii)-Catalyzed Aromatic C–H Azidation for the Synthesis of Ortho-Azido Anilines with Excellent Regioselectivity. Chem. Commun. 2014, 50, 5733–5736.
- Chen, Y.-X.; Huo, T.; Yin, Q.; Jiang, L.-F.; Cheng, X.; Ma, H.-X.; Jiang, Y.-X.; Sun, M.-Z.; Deng, Q.-H. Azidobenziodazolones as Azido Sources for the Enantioselective Copper-Catalyzed Azidation of N-Unprotected 3-Trifluoromethylated Oxindoles. Org. Lett. 2023, 25, 2739–2744.
- Yin, H.; Wang, T.; Jiao, N. Copper-Catalyzed Oxoazidation and Alkoxyazidation of Indoles. Org. Lett. 2014, 16, 2302–2305.
- Fumagalli, G.; Rabet, P.T.G.; Boyd, S.; Greaney, M.F. Three-Component Azidation of Styrene-Type Double Bonds: Light-Switchable Behavior of a Copper Photoredox Catalyst. Angew. Chem. Int. Ed. 2015, 54, 11481–11484.
- Rabet, P.T.R.; Fumagalli, G.; Boyd, S.; Greaney, M.F. Benzylic C–H Azidation Using the Zhdankin Reagent and a Copper Photoredox Catalyst. Org. Lett. 2016, 18, 1646–1649.
- Shen, K.; Wang, Q. Copper-Catalyzed Alkene Aminoazidation as a Rapid Entry to 1,2-Diamines and Installation of an Azide Reporter onto Azahetereocycles. J. Am. Chem. Soc. 2017, 139, 13110–13116.
- Sokolovs, I.; Suna, E. Para-Selective Cu-Catalyzed C–H Aryloxylation of Electron-Rich Arenes and Heteroarenes. J. Org. Chem. 2016, 81, 371–379.
- Bhattarai, B.; Tay, J.-H.; Nagorny, P. Thiophosphoramides as Cooperative Catalysts for Copper-Catalyzed Arylation of Carboxylates with Diaryliodonium Salts. Chem. Commun. 2015, 51, 5398–5401.
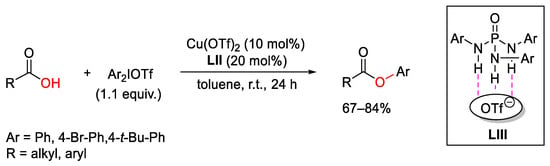
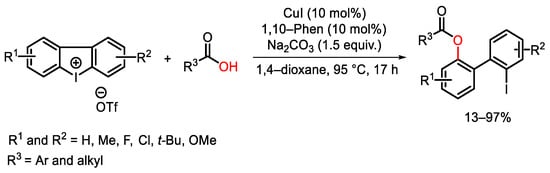
- Xie, H.; Yang, S.; Zhang, C.; Ding, M.; Liu, M.; Guo, J.; Zhang, F. Copper-Catalyzed Selective Diphenylation of Carboxylic Acids with Cycli Diaryliodonium Salts. J. Org. Chem. 2017, 82, 5250–5262.
- Zhu, K.; Xu, K.; Fang, Q.; Wang, Y.; Tang, B.; Zhang, F. Enantioselective Synthesis of Axially Chiral Biaryls via Cu-Catalyzed Acyloxylation of Cyclic Diaryliodonium Salts. ACS Catal. 2019, 9, 4951–4957.
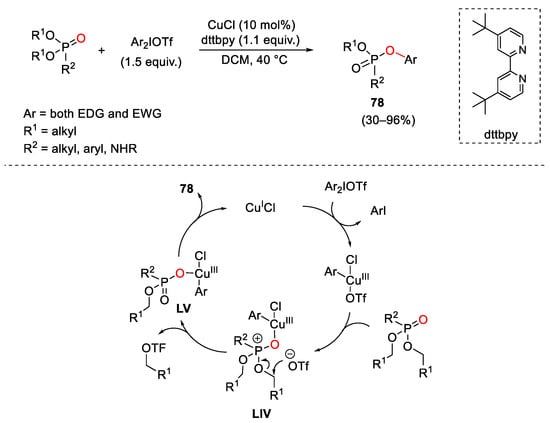
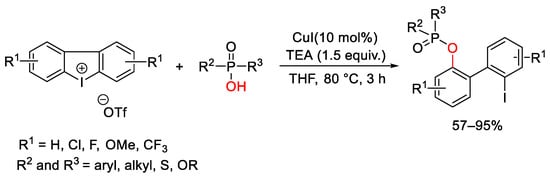
- Fañanás-Mastral, M.; Feringa, B.L. Copper-Catalyzed Synthesis of Mixed Alkyl Aryl Phosphonates. J. Am. Chem. Soc. 2014, 136, 9894–9897.
- Wang, G.; Xiong, B.; Zhou, C.; Liu, C.; Xu, W.; Yang, C.-A.; Tang, K.-W.; Wong, W.-Y. Copper-Catalyzed Diphenylation of P(O)-OH Bonds with Cyclic Diaryliodonium Salts. Chem. Asian J. 2019, 14, 4365–4374.
- Duan, L.; Zhao, K.; Wang, Z.; Zhang, F.-L.; Gu, Z. Enantioselective Ring-Opening/Oxidative Phosphorylation and P-Transfer Reaction of Cyclic Diaryliodoniums. ACS Catal. 2019, 9, 9852–9858.
- Vázquez-Galiñanes, N.; Andón-Rodríguez, M.; Gómez-Roibás, P.; Fañanás-Mastral, M. Copper-catalyzed O-alkenylation of phosphonates. Beilstein J. Org. Chem. 2020, 16, 611–615.
- Suero, M.G.; Bayle, E.D.; Collins, B.S.L.; Gaunt, M.J. Copper-Catalyzed Electrophilic Carbofunctionalization of Alkynes to Highly Functionalized Tetrasubstituted Alkenes. J. Am. Chem. Soc. 2013, 135, 5332–5335.
- Zhu, D.; Sun, Y.; Hui, P.; Li, H.; Yan, Y.; Kuang, H. Enantioselective Synthesis of Axially Chiral Oxazole Biaryls by Copper-Catalyzed Oxidation of Cyclic Diaryliodoniums. Eur. J. Org. Chem. 2022, 2022, e202101449.
- Zhu, D.; Liu, P.; Lu, W.; Wang, H.; Luo, B.; Hu, Y.; Huang, P.; Wen, S. Relayed Regioselective Alkynylation/Olefination of Unsymmetrical Cyclic Diaryliodonium Species Catalyzed by Cu and Pd: Affording Fluorescent Cytotoxic Benzoxazoles. Chem. Eur. J. 2015, 21, 18915–18920.
- Zhu, D.; Wu, Z.; Luo, B.; Du, Y.; Liu, P.; Chen, Y.; Hu, Y.; Huang, P.; Wen, S. Heterocyclic Iodoniums for the Assembly of Oxygen-Bridged Polycyclic Heteroarenes with Water as the Oxygen Source. Org. Lett. 2018, 20, 4815–4818.
- Zhu, D.; Li, M.; Wu, Z.; Du, Y.; Luo, B.; Huang, P.; Wen, S. Copper-Catalyzed One-Pot Synthesis of Dibenzofurans, Xanthenes, and Xanthones from Cyclic Diphenyl Iodoniums. Eur. J. Org. Chem. 2019, 2019, 4566–4571.
- Li, J.; Xu, Q.-N.; Wang, Z.-B.; Li, Y.; Liu, L. Synthesis of Dibenzofurans from Cyclic Diaryliodonium Triflates and Water via Oxygen–Iodine Exchange Approach. ACS Omega 2018, 3, 12923–12929.
- Luo, B.; Cui, Q.; Luo, H.; Hu, Y.; Huang, P.; Wen, S. N-Benzyldithiocarbamate Salts as Sulfur Sources to Access Tricyclic Thioheterocycles Mediated by Copper Species. Adv. Synth. Catal. 2016, 358, 2733–2738.
- Wang, M.; Wei, J.; Fan, Q.; Jiang, X. Cu(II)-catalyzed sulfide construction: Both aryl groups utilization of intermolecular and intramolecular diaryliodonium salt. Chem. Commun. 2017, 53, 2918–2921.
- Zhang, X.; Zhao, K.; Gu, Z. Transition Metal-Catalyzed Biaryl Atropisomer Synthesis via a Torsional Strain Promoted Ring-Opening Reaction. Acc. Chem. Res. 2022, 55, 1620–1633.
- Hou, M.; Deng, R.; Gu, Z. Cu-Catalyzed Enantioselective Atropisomer Synthesis via Thiolative Ring Opening of Five-Membered Cyclic Diaryliodoniums. Org. Lett. 2018, 20, 5779–5783.
- Li, B.; Chao, Z.; Li, C.; Gu, Z. Cu-Catalyzed Enantioselective Ring Opening of Cyclic Diaryliodoniums toward the Synthesis of Chiral Diarylmethanes. J. Am. Chem. Soc. 2018, 140, 9400–9403.
- Cullen, S.C.; Shekhar, S.; Nere, N.K. Cu-Catalyzed Couplings of Aryl Iodonium Salts with Sodium Trifluoromethanesulfinate. J. Org. Chem. 2013, 78, 12194–12201.
- Borrel, J.; Pisella, G.; Waser, J. Copper-Catalyzed Oxyalkynylation of C–S Bonds in Thiiranes and Thiethanes with Hypervalent Iodine Reagents. Org. Lett. 2020, 22, 422–427.
- Wang, M.; Chen, S.; Jiang, X. Construction of Functionalized Annulated Sulfone via SO2/I Exchange of Cyclic Diaryliodonium Salts. Org. Lett. 2017, 19, 4916–4919.
- Yang, Y.-D.; Azuma, A.; Tokunaga, E.; Yamasaki, M.; Shiro, M.; Shibata, N. Trifluoromethanesulfonyl Hypervalent Iodonium Ylide for Copper-Catalyzed Trifluoromethylthiolation of Enamines, Indoles, and β-Keto Esters. J. Am. Chem. Soc. 2013, 135, 8782–8785.
- Duan, L.; Wang, Z.; Zhao, K.; Gu, Z. Enantioselective preparation of atropisomeric biaryl trifluoromethylsulfanes via ring-opening of cyclic diaryliodoniums. Chem. Commun. 2021, 57, 3881.
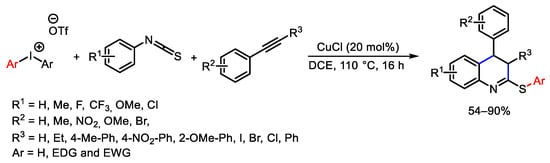
- Khan, S.; Volla, C.M.R. Cu-catalyzed Cascade Cyclization of Isothiocyanates, Alkynes, and Diaryliodonium Salts: Access to Diversely Functionalized Quinolines. Chem. Eur. J. 2017, 23, 12462–12466.
- Wang, W.; Zhou, J.; Wang, C.; Zhang, C.; Zhang, X.-Q.; Wang, Y. Design, development and applications of copper-catalyzed regioselective (4+2) annulations between diaryliodonium salts and alkynes. Commun. Chem. 2022, 5, 145.
- Liu, Z.; Zhu, D.; Luo, B.; Zhang, N.; Liu, Q.; Hu, Y.; Pi, R.; Huang, P.; Wen, S. Mild Cu(I)-Catalyzed Cascade Reaction of Cyclic Diaryliodoniums, Sodium Azide, and Alkynes: Efficient Synthesis of Trazolophenanthridines. Org. Lett. 2014, 16, 5600–5603.
- Urones, B.; Martínez, A.M.; Rodríguez, N.; Arrayás, R.G.; Carretero, J.C. Copper-catalyzed ortho-halogenation of protected anilines. Chem. Commun. 2013, 49, 11044–11046.
- Giofré, S.; Loro, C.; Molteni, L.; Castellano, C.; Contini, A.; Nava, D.; Broggini, G.; Beccalli, E.M. Copper(II)-Catalyzed Aminohalogenation of Alkynyl Carbamates. Eur. J. Org. Chem. 2021, 2021, 1750–1757.
- Hao, W.; Liu, Y. C-H bond halogenation catalyzed or mediated by copper: An overview. Beilstein J. Org. Chem. 2015, 11, 2132–2144.
- Yuan, W.; Szabò, K.J. Catalytic Intramolecular Aminofluorination, Oxyfluorination, and Carbofluorination with a Stable and Versatile Hypervalent Fluoroiodine Reagent. Angew. Chem. Int. Ed. 2015, 54, 8533–8537.
- Parvathaneni, S.P.; Perumgani, P.C. Regioselective Chlorination of Aryl C-H bonds with Hypervalent Iodine(III) Reagent 1-Chloro-1,2-benziodoxol-3-one. Asian J. Org. Chem. 2018, 7, 324–327.
- Wu, B.; Yoshikai, N. Conversion of 2-Iodobiaryls into 2,2’-Diiodobiaryls via Oxidation- Iodination Sequences: A Versatile Route to Ladder-Type Heterofluorenes. Angew. Chem. Int. Ed. 2015, 54, 8736–8739.
- Ke, J.; Zu, B.; Guo, Y.; Li, Y.; He, C. Hexafluoroisopropanol-Enabled Copper-Catalyzed Asymmetric Halogenation of Cyclic Diaryliodoniums for the Synthesis of Axially Chiral 2,2’-Dihalobiaryls. Org. Lett. 2021, 23, 329–333.
- Xu, J.; Zhang, P.; Gao, Y.; Chen, Y.; Tang, G.; Zhao, Y. Copper-Catalyzed P-Arylation via Direct Coupling of Diaryliodonium Salts with Phosphorus Nucleophiles at Room Temperature. J. Org. Chem. 2013, 78, 8176–8183.
- Beaud, R.; Phipps, R.J.; Gaunt, M.J. Enantioselective Cu-Catalyzed Arylation of Secondary Phosphine Oxides with Diaryliodonium Salts toward the Synthesis of P-Chiral Phosphines. J. Am. Chem. Soc. 2016, 138, 13183–13186.
- Cho, S.H.; Yoon, J.; Chang, S. Intramolecular Oxidative C-N Bond for the Synthesis of Carbazoles: Comparison of Reactivity between the Copper-Catalyzed and Metal-Free Conditions. J. Am. Chem. Soc. 2011, 133, 5996–6005.
- Mirallai, S.I.; Koutentis, P.A. The Conversion of 4-Anilinquinazoline- and 3-Aryl-4-imino-3,4-dihydro-quinazoline-2-carbonitriles into Benzoimidazoquinazoline-6-carbonitriles via Oxidative and Nonoxidative C-N Couplings. J. Org. Chem. 2015, 80, 8329–8340.
- Liu, Z.-J.; Lu, X.; Wang, G.; Li, L.; Jiang, W.-T.; Wang, Y.-D.; Xiao, B.; Fu, Y. Directing Group in Decarboxylative Cross-Coupling: Copper-Catalyzed Site-Selective C-N Bond Formation from Nonactivated Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2016, 138, 9714–9719.
- Yang, Y.-N.; Jiang, J.-L.; Shi, J. Mechanistic Study of Copper-Catalyzed Decarboxylative C-N Cross-Coupling with Hypervalent Iodine Oxidant. Organometallics 2017, 36, 2081–2087.
- Yuan, J.; Rao, C.B.; Liang, Y.; Zhang, R.; Zhang, Q.; Hou, L.; Dong, D. Copper-Catalyzed Regioselective Oxidation Cycloamidation of α--Alkylamides: Synthetic Route to Substituted Pyrrolidine-2,4—Diones. Adv. Synth. Catal. 2019, 361, 160–169.
- Moreau, B.; Alberico, D.; Lindsay, V.N.G.; Charette, A.B. Catalytic asymmetric synthesis of nitrocyclopropane carboxylates. Tetrahedron 2012, 68, 3487–3496.
- Chidley, T.; Murphy, G.K. Cyclopropanation of alkenes with metallocarbenes generated from monocarbonyl iodonium ylides. Org. Biomol. Chem. 2018, 16, 8486–8490.
- Wan, J.-P.; Lin, Y.; Cao, X.; Liu, Y.; Wei, L. Copper-catalyzed, hypervalent iodine mediated C=C bond activation of enaminones for the synthesis of α-keto-amides. Chem. Commun. 2016, 52, 1270–1273.
- Loro, C.; Molteni, L.; Papis, M.; Beccalli, E.M.; Nava, D.; Lo Presti, L.; Brenna, S.; Colombo, G.; Foschi, F.; Broggini, G. Direct Synthesis of Fluorescent Oxazolo-phenoxazines by Copper-Catalyzed/Hypervalent Iodine(III)-Mediated Dimerization/Cyclization of 2-Benzylamino-phenols. J. Org. Chem. 2022, 87, 1032–1042.
- Liu, Z.; Huang, F.; Lou, J.; Wang, Q.; Yu, Z. Copper-promoted direct C-H alkoxylation of S,S-functionalized internal olefins with alcohols. Org. Biomol. Chem. 2017, 15, 5535–5540.
More
