Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dana Antonia Tapoi and Version 2 by Rita Xu.
Cutaneous melanoma (CM) is an increasingly significant public health concern. Due to alarming mortality rates and escalating incidence, it is crucial to understand its etiology and identify emerging biomarkers for improved diagnosis and treatment strategies.
- cutaneous melanoma
- immunohistochemistry
- genetic mutations
1. Introduction
Cutaneous melanoma (CM) is a particularly aggressive form of cancer that originates from melanocytes, pigment-producing cells derived from the neural crest [1]. Despite representing a mere 4% of all skin cancers, CM accounts for up to 75% of skin cancer-related deaths [2]. However, with early detection and proper intervention, over 90% of the cases could be cured [3].
The pathogenesis of CM is multifactorial, involving both genetic and environmental factors [4]. Ultraviolet radiation (UVR), either from natural light or artificial sources, is the most important environmental risk factor for CM. Additionally, individuals with lighter complexion have the highest risk of developing CM due to lower levels of melanin which make these individuals more likely to develop sunburns. A higher number of nevi are also associated with an increased risk. A familial history of CM further increases this risk, possibly due to shared sun exposure behaviors or hereditary genetic mutations [5].
Patient survival is strongly correlated with an early detection of the disease. Among various prognostic factors, the depth of invasion remains the most critical determinant of survival in numerous studies. Intraepidermal (in situ) melanomas can be cured by excision alone, and thin melanomas have minimal metastatic potential [6]. On the contrary, thick CMs still have very high mortality rates [7].
2. Melanoma Pathogenesis
Ultraviolet radiation exposure is a major risk factor for CM due to the UVR capacity to damage DNA, causing somatic mutations [8]. The exposure can be classified as intermittent or chronic, the latter being mostly occupational. Both these exposure patterns are associated with an increased risk of CM, but it appears that the risk is higher for intermittent exposure [8][9][8,9]. This may be explained by the fact that intermittently exposed individuals have lower melanin levels and are more likely to develop sunburns [10]. Nevertheless, there are cutaneous melanomas, such as acral melanomas, which arise in skin that is not exposed to UVR. In this context, according to the 2023 WHO Classification of Tumors, cutaneous melanomas are classified as melanomas arising in sun-exposed skin and melanomas arising in sun-shielded sites (Table 1) [11].Table 1. WHO Classification of cutaneous melanomas.
| Melanomas arising in sun-exposed skin | Low CSD melanoma: SSM, low CSD nodular melanoma |
| High CSD melanoma: lentigo malignant melanoma, high CSD nodular melanoma | |
| Desmoplastic melanoma: most often associated with severely sun-damaged skin | |
| Melanomas arising in sun-shielded skin or without known UVR exposure | Spitz melanoma |
| Acral melanoma | |
| Melanoma arising in congenital nevus | |
| Melanoma arising in blue nevus |
3. Emerging Biomarkers in CM
Due to the extraordinarily heterogenous histopathological, immunohistochemical, and molecular landscape of CM, this disease continues to pose important challenges in terms of diagnosis and treatment. Therefore, several new biomarkers have become increasingly studied in recent years in the hope of improving the understanding of CM pathogenesis and management. Unlike immunohistochemical and genetic testing, these emerging biomarkers are expected to improve the early detection and subsequent monitoring of CM in rapid, cost-effective, and non-invasive ways as they can easily be analyzed from blood samples. Furthermore, these new biomarkers may also serve as potential therapeutic targets.3.1. MicroRNA
MicroRNA (miRNAs) represent non-coding RNAs involved in degrading mRNAs [58][96]. They have been increasingly recognized as critical modulators of oncogenic processes, including various stages of cancer progression such as melanoma [59][60][111,112]. In melanomas, miRNA dysregulation is involved in promoting cell proliferation, resistance to apoptosis and invasion, angiogenesis, and metastasis [61][113]. Additionally, miRNAs have also been associated with resistance to BRAF and MAPK inhibitors [62][63][114,115]. In this context, due to their detectability in both intra- and extracellular compartments and their stable levels even in unfavorable conditions, miRNAs have attracted considerable attention as emerging biomarkers in oncology [64][116]. At present, miRNA levels can be assessed from various sources, such as resected primary or metastatic tumors, as well as arterial or venous plasma and serum. Importantly, the data derived from these different sources have shown no significant divergence. Depending on the type of cancer under investigation, abnormal miRNA expression profiles have been found to correlate with various disease stages, overall prognosis, tumor recurrence, and potential responsiveness to therapeutic interventions [59][65][111,117]. In patients with melanoma, different miRNAs can be either up- or down-regulated, and have been correlated with progression-free survival and overall survival [66][67][68][118,119,120]. Interestingly, serum levels of various microRNAs can discriminate between melanoma stages with increased accuracy compared to S100B or LDH [69][121]. For instance, miR-137, miR-148, and miR-182 downregulate MITF expression and promote tumor invasion [70][122]. miR-221 plasma levels are increased in melanoma patients, and are correlated with stage, recurrence, and disease progression [71][123]. Rigg E. et al. demonstrated that miR-146a-5p is overexpressed in melanoma brain metastases and its knockdown results in a reduction of metastatic lesions [72][124]. On the contrary, other types of microRNA such as mirR-211, miR-542 3p, or miR-152-3p are downregulated in invasive melanomas [73][125]. In vitro studies demonstrated that increasing miR-152-3p expression inhibits the proliferation and invasiveness of melanoma cells [74][126]. miR-542 3p is involved in epithelial-to-mesenchymal transition (ETM), and its experimental upregulation inhibited ETM and metastatic spread [75][127]. miR-143 also bears anti-tumoral effects as it has been linked to promoting apoptosis and inhibiting the proliferation of melanoma cells [76][128]. Similar anti-tumoral effects have been reported for miR-224-5p which can additionally overturn acquired resistance to BRAF inhibitors [77][129]. Several other microRNA seem to play a role in resistance to target or conventional chemotherapy. A downregulation of miR-7, miR-579 3p, and miR-126 3p was found in melanomas resistant to BRAF/MAPK inhibitors [78][79][80][130,131,132], while miR-31 downregulation is associated with chemoresistance [81][133]. Importantly, the experimental upregulation of miR-7 and miR-126 3p restored responses to BRAF inhibitors in melanoma cell lines [78][79][130,131].3.2. Exosomes
Exosomes are extracellular vesicles secreted by cells, encompassing a unique molecular signature that reflects the cell type from which they originate. Given their traceable cellular origins and facile isolation, exosomes can be regarded as potential biomarkers for diagnosis and prognosis in various cancers, including melanoma [82][134]. They are readily available through minimally invasive methods, as they can be isolated from a variety of biological fluids such as blood, plasma, urine, and cerebrospinal fluid [83][135]. Exosomes extracted from melanoma cell lines have been shown to contain distinct mRNA, miRNA, and protein profiles [82][83][134,135]. Exosome analysis can offer important diagnostic and prognostic information, as various exosomal components are significantly altered in cutaneous melanomas [82][84][85][134,136,137]. In this respect, Surman M. et al. found increased exosome concentrations in melanoma cases but those levels were not correlated with disease stage [82][134]. On the contrary, Boussadia Z. et al. reported a higher exosome concentration in metastatic melanoma compared to non-metastatic cases [86][138]. The complex relationship between exosomal components and melanoma progression is not entirely understood, but various mechanisms have been proposed. For instance, exosomes can carry and modulate the activity of matrix metalloproteinases (MMPs), as well as alter cell adhesion and activate fibroblasts to become cancer-associated fibroblasts, thus stimulating melanoma invasiveness [82][87][88][89][134,139,140,141]. Exosomal components have also been shown to enhance metastatic potential in CM by promoting epithelial-to-mesenchymal transition (ETM) [90][142], angiogenesis [82][91][134,143], and lymphangiogenesis [92][144]. As melanoma is particularly prone to brain metastases, exosomes may also at least partially explain this characteristic by damaging endothelial cells and the blood–brain barrier, and activating glial cells [93][145]. Some exosomal components have also been linked to resistance to therapy in CM, and targeting these molecules may improve therapeutic response [94][146]. In this context, exosomes can influence the melanoma microenvironment by altering the function of lymphocytes and stimulating tumor-associated macrophages (TAMs) to become M2-polarized and secrete pro-tumorigenic cytokines [95][96][97][147,148,149]. These effects can affect the response to immunotherapy, and targeting TAMs could improve the outcome of melanoma patients [98][150]. Furthermore, exosomes can be used as a means for administering therapy [99][151]. In this respect, exosomes containing BRAF siRNA were shown to have increased anti-tumoral activity compared to siBRAF in melanoma cell lines [100][152]. Similarly, cord-blood-derived exosomes produced significant genotoxicity and a decrease in survival time for melanoma cells and lymphocytes from melanoma patients, apparently by delivering anti-oncogenic miR-7. These results are particularly important as the exosome caused no significant damage to normal lymphocytes [101][153]. While these findings underscore the promising role of exosomes as diagnostic, prognostic, and treatment tools in melanoma, additional research is required to comprehensively delineate their utility. Future studies may aim to validate these biomarkers in larger patient cohorts, completely elucidate the roles of exosomal components in melanoma progression, and assess the feasibility of incorporating exosomal markers into existing diagnostic, prognostic, and therapeutic frameworks. The complex effects of exosomal components in CM pathogenesis are presented in Figure 1.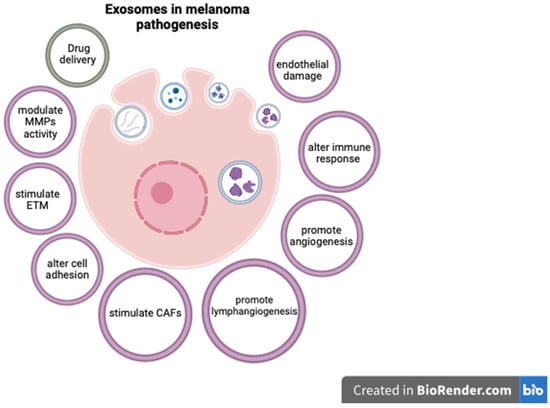
Figure 1. The role of exosomal components in CM.
