You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Mohit Mathur and Version 2 by Sirius Huang.
A PRoliferation-Inducing Ligand (APRIL), the thirteenth member of the tumor necrosis factor superfamily, plays a key role in the regulation of activated B cells, the survival of long-lived plasma cells, and immunoglobulin (Ig) isotype class switching. Several lines of evidence have implicated APRIL in the pathogenesis of IgA nephropathy (IgAN).
- APRIL
- A PRoliferation-Inducing Ligand
- B cells
- BCMA
- BAFF
- IgA nephropathy
- TACI
- TNFSF13
1. Introduction
The thirteenth member of the tumor necrosis factor superfamily (TNFSF), designated A PRoliferation-Inducing Ligand (APRIL), was first described in 1998 as a molecule with the ability to stimulate tumor cell growth [1]. It is closely related to the B-cell growth factor BAFF (B-cell activating factor of the tumor necrosis factor [TNF] family), with APRIL and BAFF sharing ~30% sequence homology within the TNF domain [2]. Both APRIL and BAFF are able to bind to the TNF receptor transmembrane activator calcium modulator and cyclophilin ligand (CAML) interactor (TACI) and B-cell maturation antigen (BCMA) [3]; however, BAFF differs from APRIL in its ability to bind to a specific receptor, BAFF-R [4].
Despite the similarities observed between APRIL and BAFF, the two cytokines work via different molecular pathways and have differing physiologic roles. A genome-wide association study (GWAS) identified APRIL as a key susceptibility locus for IgA nephropathy (IgAN) [5], which is a chronic and progressive condition resulting from abnormal O-glycosylation of IgA1 and the deposition of immune complexes in the kidneys, leading to inflammation and damage [6]. APRIL has also been implicated in several other autoimmune conditions, including systemic lupus erythematosus (SLE), rheumatoid arthritis, alopecia areata, myasthenia gravis, Sjögren’s syndrome, and bullous pemphigoid [7]. APRIL and BAFF are both involved in immunoglobulin (Ig) class switching in B cells, thereby contributing to the pathogenesis of disorders with aberrant Ig production [8]. In addition, APRIL, BAFF, and their receptors are reported to be expressed at high levels in various cancers and appear to be associated with disease severity and treatment response [4].
The functional heterogeneity between APRIL and BAFF in both normal and pathological processes makes them independent candidates for therapeutic intervention. While the role of BAFF in B-cell maturation has made it an obvious target for disease interventions [9][10][9,10], research has also begun to elucidate the importance of APRIL, particularly in autoimmune diseases [7]. Specifically, a large body of research has served to corroborate the association between APRIL and IgAN [11][12][11,12], paving the way for the development of novel therapies for this potentially life-threatening condition.
2. The Biological Roles of APRIL in Health and Disease
2.1. APRIL Production
The gene encoding APRIL is located on chromosome 17p13, and the resultant protein is predominantly produced by myeloid cells (monocytes, macrophages, and dendritic cells) and T cells [13]. Normal APRIL expression is induced during bone marrow hematopoiesis [14], but it can also be stimulated in the epithelial cells of the gut, tonsils, and skin [15]. APRIL is synthesized as a type II transmembrane protein and requires intracellular cleavage and processing within the Golgi apparatus, before being secreted in its biologically active form [16].
2.2. Physiologic Functions
2.2.1. B-Cell Survival
APRIL, BAFF, and their receptors are known to have specific functions in the process of B-cell maturation and survival. Each step of B-cell maturation within the bone marrow is dependent on APRIL or BAFF, including the development into functional but immature cells, migration to the spleen, antigen encounter, and differentiation into either antibody-secreting cells (ASCs) or antigen-presenting cells (APCs) [17][18][17,18].
APRIL binds strongly to BCMA and with lower affinity to TACI, while soluble BAFF binds strongly to TACI and BAFF-R, but with weak affinity to BCMA [17]. In addition, APRIL, but not BAFF, is able to bind to cell-surface proteoglycans, which may increase localized APRIL concentration and signaling [17]. Both APRIL and BAFF stimulate B-cell proliferation, but while BAFF is needed for the development of mature B cells, APRIL is involved in the plasma cell survival in the bone marrow [19]. The two cytokines also have independent and non-overlapping roles in Ig isotype class switching in B cells [20][21][20,21]. (Table 1).
Table 1.
Overview of BAFF and APRIL functions by receptor type.
| Function | APRIL | BAFF | Receptors Mediating These Functions | |||
|---|---|---|---|---|---|---|
| TACI | BCMA | BAFF-R | Proteoglycans | |||
| B-cell survival and maturation | X | X | X | X | ||
| Plasma blast and plasma cell differentiation and survival | X | X | ||||
| Maintenance of B1 cell | X | X | ||||
| T-cell-independent antibody response | X | X | X | X | X | |
| Antibody class switch and recombination | X | X | ||||
| Plasma cell trafficking | X | X | ||||
X; data are available to support this function. [4][13][22][23][4,13,22,23] APRIL, A PRoliferation-Inducing Ligand; BAFF, B-cell activating factor of the tumor necrosis factor family; BAFF-R, BAFF receptor; BCMA, B-cell maturation antigen; TACI, transmembrane activator calcium modulator and cyclophilin ligand interactor.
The binding of BAFF to BAFF-R is critical for mature B-cell development, as demonstrated by the fact that mice deficient in active BAFF have impaired differentiation and a deficit in mature B cells, resulting in impaired humoral responses to antigens [24]. In contrast, primary B-cell maturation in the spleen is not impaired in mice over- or under-expressing APRIL [25][26][25,26], but APRIL is necessary to regulate activated B cells and plays a key role in the survival of long-lived plasma cells in bone marrow [19][27][19,27].
2.2.2. Ig Class Switching
Another role for APRIL and BAFF is in Ig isotype class switching in B cells [20][21][20,21]. The two proteins have non-overlapping roles in this process, whereby class switching can be independently induced via either APRIL–TACI or BAFF–BAFF-R/BAFF–TACI binding [28]. APRIL–TACI and APRIL–proteoglycan binding also contribute to IgA production modulation by B cells and antibody responses to T-cell-dependent antigens [29].
As a B-cell survival factor with the ability to induce T-cell-independent Ig class switching, APRIL plays a key role in the maintenance of the mucosal immunologic barrier [30]. Intestinal epithelial cells produce APRIL and APRIL-inducing cytokines after sensing bacteria through Toll-like receptors. In human IgA1-expressing B cells originating from Peyer’s patches, APRIL triggers sequential class switching to IgA2. This switch enriches the distal intestinal tract with IgA2, which is more resistant to bacterial degradation than IgA1 [31].
APRIL plays a key role in modulating the gut mucosal immune axis, and the hypothesis that the dysfunction of this axis may play a key role in the pathogenesis of IgAN is discussed in more detail in Section 4.2.
2.2.3. Downstream Effects on T Cells
APRIL does not directly contribute to T-cell activation, but its downstream signaling pathways have been implicated in the activation of CD4+ cells [32]. Furthermore, one research group has demonstrated that APRIL knockout mice have a greater number of effector memory T cells, enlarged germinal centers, and elevated IgG responses to T-dependent antigens, despite having normal T- and B-cell development [33].
2.3. Association with Disease
Unsurprisingly, given their key roles in B-cell homeostasis and survival, both APRIL and BAFF have also been implicated in the development or maintenance of a myriad of diseases [34]. Aberrant expression levels of APRIL and BAFF have been reported in association with various pathologies, including cancer [4], immunodeficiency [35][36][35,36] or autoimmune [37][38][37,38] diseases, infection [39][40][39,40], and allergies [41].
The disruption of B-cell tolerance may be one potential mechanism underlying the role of APRIL in pathologic diseases, particularly immune-related conditions. The plasmablasts and fully differentiated plasma cells responsible for producing autoantibodies are likely to be regulated by APRIL signaling, and APRIL expression has been reported to be associated with the severity and progression of several autoimmune diseases [7][42][7,42].
In patients with SLE, the aberrant production of APRIL by B cells has been reported [43], in addition to the normal cellular expression by myeloid cells. Furthermore, this uncharacteristic APRIL production could be reproduced by exposing healthy cells to toll-like receptor ligands, which could potentially indicate a B-cell autocrine pathway leading to autoantibody production [44]. Seropositive patients with rheumatoid arthritis have also been reported to have high serum concentrations of APRIL, which correlate with disease activity [45]. Localized APRIL upregulation in the synovial fluid of patients [45] may be a result of the production by local myeloid cells and synovial fibroblasts [46]. Reports have linked increased APRIL serum concentrations with a multitude of other autoimmune diseases [7], and there are also several strands of evidence, including clinical reports, genomic data, and the results from animal models; these have connected alterations in APRIL expression and signaling with the development and severity of IgAN, as detailed below.
3. Overview of IgAN
IgAN is considered to be the most common primary glomerular disease worldwide, with estimates placing the overall global incidence at ≥2.5 per 100,000 individuals [47]. IgAN incidence varies by race, ethnicity, and geographic region; Asian populations appear to have a greater risk of developing IgAN compared with Caucasian populations [48][49][50][48,49,50], with up to 60% of biopsy-diagnosed glomerular disease in Asian countries attributed to IgAN [51]. In contrast, the proportion in Europe is around 30%, and in the US, it is about 10% [51]. There is also considerable heterogeneity reported in the clinical manifestations and outcomes between individuals of different races and ethnicities [52][53][52,53]. Diagnostic confirmation of IgAN requires a kidney biopsy [54], and once diagnosed, patients could progress to kidney failure within 15 years [55].
Although aspects of the underlying pathophysiology remain unclear, the development of IgAN can be described by a 4-hit hypothesis (Figure 12). Hit 1 is an increase in the production and circulation of galactose-deficient IgA1 (Gd-IgA1), which results in the production of autoantibodies (hit 2) against Gd-IgA1. Immune complexes consisting of Gd-IgA1 and autoantibodies then form and circulate (hit 3) and are deposited within the glomerular mesangium (hit 4) [56][57][56,57]. Subsequently, cytokine production at the sites of the immune complex deposition result in localized inflammation and activation of the complement system, renin angiotensin system, and stimulation of mesangial proliferation [58].
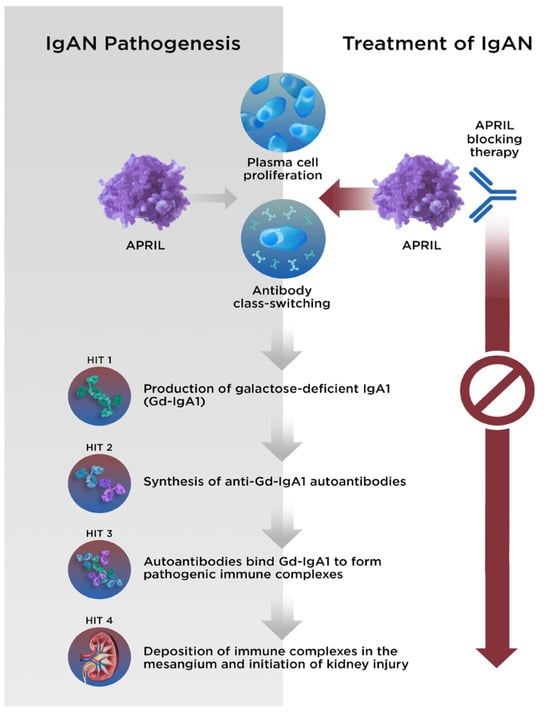
Figure 12. The 4-hit hypothesis describes the pathogenesis of IgAN. With APRIL-blocking therapy, each stage of disease can be halted. APRIL, A PRoliferation-Inducing Ligand; Gd-IgA1, galactose-deficient immunoglobulin A1; IgA1, immunoglobulin A1; IgAN, immunoglobulin A nephropathy.
IgA antibodies provide the first line of defense against infection at mucosal surfaces, neutralizing bacterial and viral pathogens and maintaining mucosal homeostasis [59]. IgA1 has a longer hinge region than IgA2. Changes in the O-glycosylation of the serine and threonine residues in the hinge region of IgA1 result in the formation of Gd-IgA1 [56][59][56,59]. Interestingly, heterogeneity in the O-glycoforms contained within the IgA1 hinge-region has been reported between Asian and Caucasian patients and may partially account for the increased susceptibility to IgAN in Asian populations [60]. Once Gd-IgA1 has been generated, the circulating levels have been shown to relate to worsened prognoses and outcomes in patients with IgAN [61][62][61,62].
4. Evidence Supporting the Involvement of APRIL in IgAN
4.1. Gut Mucosa–Kidney Axis in IgAN
Gd-IgA1 is thought to be produced by ASCs located in the mucosa-associated lymphoid tissue, particularly the gut-associated lymphoid tissue (GALT) [54]. IgA found in mesangial deposits in the kidneys of patients with IgAN includes the polymeric secretory form exclusively produced at the mucosal surface [56]. Moreover, although challenging to study, there is considerable evidence for a ‘gut-kidney axis’ in kidney diseases, with both the gut microbiome and diet reported to have an impact on the development and progress of IgAN [57][58][63][57,58,63]. An exaggerated IgA response to mucosal antigen challenge has been reported in IgAN, and it is thought that alimentary pathogens or antigens may initiate aberrant mucosal B-cell activation and Gd-IgA1 synthesis [64]. Notably, while no single pathogenic organism has been found to be associated with IgAN, it seems that a dysfunctional response to commensal organisms, driven via APRIL and/or BAFF signaling, may play a critical role [65][66][65,66].
4.2. APRIL Is Produced in GALT
The examination of tissue from normal human gut has shown that APRIL is expressed in GALT, lamina propria, and intestinal epithelium, with particularly strong expression in areas located close to lymphoid tissue [30]. It has been hypothesized that in healthy individuals, APRIL assists in maintaining the mucosal barrier and promotes the survival of mucosal plasma cells [30]. It is also involved in IgA class switch recombination via its binding to TACI [67]; notably, while both APRIL–TACI and BAFF–TACI binding have been shown to induce IgA class switching in vitro, only APRIL appears to have this effect in vivo [68].
4.3. Clinical Epidemiology
The reports in the published literature have demonstrated that the normal physiologic roles of APRIL (IgA class switching and the survival of IgA-producing plasma cells [30][67][30,67]) are implicated in the pathophysiology of IgAN, whereby gut hyperresponsiveness and elevated APRIL expression result in the increased production of Gd-IgA1, thus providing a critical link to hit 1 of the 4-hit mechanism of IgAN [54].
Increased levels of APRIL have been observed in several clinical studies of IgAN, correlating with both Gd-IgA1 levels and disease severity (increased proteinuria and decreased estimated glomerular filtration rate [eGFR]) [56]. In a comparative study of 166 patients with IgAN and 77 healthy controls, elevated plasma levels of APRIL were observed in IgAN, accompanied by increased TACI and BCMA expression in B cells and overproduction of Gd-IgA1 [15]. In another study, B cells from patients with IgAN were found to have increased expression of Gd-IgA1 (but not total IgA) when challenged with recombinant human APRIL, while blockage of TACI and BCMA abrogated these effects [69]. Moreover, the plasma levels of APRIL in patients are associated with both the rate of eGFR loss [70] and the risk of progression to end-stage kidney disease [69].
4.3.1. Debate 1: IgAN Pathology: APRIL, BAFF, or Both?
Data from a recent Canadian study confirmed elevated median levels of APRIL in patients with IgAN versus nonrelated household-matched control participants (1.98 ng/mL vs. 1.55 ng/mL; p < 0.01), and a positive correlation between serum levels of APRIL and proteinuria (Spearman’s rho = 0.28; p = 0.01) [71]. Notably, however, no differences in BAFF levels were observed between groups [71]. In Spanish patients with IgAN who underwent kidney transplantation, an increase in APRIL was found to precede IgAN recurrence, while the BAFF levels remained unchanged [72].
Conversely, other researchers, in China [73] and Italy [74] have reported elevated BAFF levels in patients with IgAN versus healthy controls. Additional, larger-scale studies are needed to determine the true picture and whether there may be demographic (e.g., race/ethnicity) or disease-related characteristics that could account for this discrepancy.
Current drug development is split between agents that inhibit either one or both pathways. However, caution is required when administering agents that have a profound impact on the immune system, due to the potential for opportunistic infections [75]. This risk is likely to be higher when inhibiting both APRIL and BAFF, since altering both B-cell maturation (BAFF) and the survival of mature plasma cells (APRIL) may further escalate the safety risks. A study with a dual APRIL/BAFF inhibitor in patients with SLE reported that the incidence of treatment-emergent adverse events and infections was comparable between the two groups; however, the levels of CD19+ B cells were reduced by approximately 50% following treatment [76], which could be a safety liability for patients. Hypothetically, however, APRIL inhibition may impart less risk than dual inhibition, as APRIL inhibition does not impede B-cell maturation; thus, the pool of mature B cells and the intact T-cell responses are maintained.
4.3.2. Debate 2: Does Nasopharyngeal Lymphoid Tissue Play a Major Role?
In addition to GALT, nasal-associated lymphoid tissue (NALT) in the tonsils and adenoids has also been implicated in the pathogenesis of IgAN. Plasma cells in NALT produce a greater ratio of IgA1 to IgA2 [77], and a link between oral and tonsillar microbiota and dysregulated NALT immunoreactivity has been postulated as a cause of IgAN [78][79][78,79]. Mass spectrometry data demonstrated that the IgA1 produced by ASCs in the tonsils was galactose deficient in the hinge region [80]. In a study of 24 patients with IgAN, the cells in the tonsillar germinal centers produced APRIL, indicating an upregulation of APRIL expression in this region compared with that of the control patients [81]. Moreover, this aberrant APRIL expression correlated with proteinuria [81].
In Japan and other Asian countries, tonsillectomy has been implemented within the treatment regimen for IgAN for the past two decades, with multiple studies reporting beneficial outcomes [82][83][84][85][82,83,84,85]. However, patients in other geographic regions have not shown the same response [86], and tonsillectomy is not routinely recommended for Caucasian patients with IgAN [87].
4.4. Insights from Genetic Studies
Among the GWAS conducted to search for susceptibility loci underlying IgAN, several studies have primarily served to confirm a link between race/ethnicity and disease by indicating an association with human leukocyte antigen variants [88][89][88,89]. However, a consistent association between IgAN and the chromosomal region 17p13 (encoding APRIL) has also been reported in GWAS studies, with this association remaining valid across patients with either European or East Asian ancestry [5][90][5,90]. Of note, although one GWAS suggested the involvement of BAFF in B-cell immune responses in tonsillectomy samples [91], BAFF was not identified as a locus of interest in either of the IgAN GWAS in which APRIL was detected. In another GWAS of serum protein levels in Japanese individuals, variants at the 17p13 locus of the APRIL gene and at the 17p11 locus encoding TACI were associated with levels of total protein, non-albumin protein, and immunoglobulins (including IgA) [92]. Similarly, in patients with IgAN, those with the 17p23 risk variant were found to have elevated serum IgA levels [5], further supporting the link between APRIL and IgAN pathophysiology.
