Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yonggang MENG and Version 2 by Jessie Wu.
Tribotronics represents the modulation of friction via an external electric potential, a field with promising ramifications for intelligent devices, precision manufacturing, and biomedical applications. A profound elucidation of mechanisms that allow for potential-controlled friction is foundational to further research in this tribotronic domain.
- tribotronic
- interfacial interaction
- redox
- physisorption
- structure transformations
1. Introduction
Friction is ubiquitous in daily life and industrial manufacturing, arising when two contact surfaces slide against one another. This interaction can lead to wear and tear on machinery and equipment, potentially causing premature failure or diminished accuracy [1][2][1,2]. Yet, friction remains integral to human activities and productivity. For instance, locomotion in humans and animals necessitates friction between limbs and terrain, just as the operation of vehicles and railways hinges on friction for torque transmission. In recent years, the increasing sophistication and digitalization of electromechanical products have necessitated not only the reduction or enhancement of friction but also its real-time active control. Designing a high-precision, rapidly adjustable friction control system is paramount for advancing cutting-edge equipment. Active friction control methods encompass load adjustments [2][3][2,3], lubricant application [4][5][6][7][8][4,5,6,7,8], and external field manipulation [9][10][9,10]. While load modifications necessitate supplementary control mechanisms, increasing machinery complexity, lubricants can reduce friction between moving surfaces but fail to offer reversible friction control or preset friction coefficient (COF) magnitudes. By contrast, external field stimulation allows for reversible active friction control without added mechanical intricacy. Common external field control techniques include electric potential control [11][12][11,12], magnetic field manipulation [13][14][15][13,14,15], and temperature regulation [16][17][18][16,17,18]. Of these methods, electric potential control is regarded as the most promising approach for achieving high-precision and rapid active friction control [19][20][19,20]. This is attributed to the real-time adjustability of electrode surface properties in potential control systems, enabling swift transitions in friction characteristics. Furthermore, in an electrochemical system maintaining constant potential, electrode surface properties can be preserved for extended periods, offering the potential for precise friction control through voltage manipulation.
The Stribeck curve delineates three distinct lubrication regimes: hydrodynamic, mixed, and boundary lubrication. Hydrodynamic lubrication and boundary lubrication represent two quintessential states of lubrication [21][22][21,22], as depicted in Figure 1. In the state of hydrodynamic lubrication, two solid surfaces are entirely separated by a consistent fluid film [23], with the external load being fully supported by this film. Predominantly, friction in this lubrication state arises due to the shear force existing between the solid surfaces and the fluid film, with the viscosity of the fluid film playing a pivotal role in determining friction. The utilization of electromagnetically responsive fluid lubricants under electric or magnetic fields can facilitate reversible control of the interfacial friction [24][25][26][27][24,25,26,27]. This control is achieved through alterations in the fluid’s viscosity, which are induced by structural transformations of fluid molecules under the influence of electromagnetism. In the state of boundary lubrication, the solid surface is covered with a boundary lubrication film of single or multiple molecular thicknesses, which is either physically or chemically adhered. The external load is supported either by the boundary lubrication film itself and the distributed asperity peaks that penetrate the film (on a rough surface) or solely by the boundary lubrication film (on an atomically smooth surface) [21][28][21,28]. For rough surfaces, the interfacial friction is primarily governed by the shear stress of the boundary lubrication film and the asperity peak surfaces. It is important to note that asperity peaks, being inherently solid materials, generate significant shear stress. Conversely, the boundary lubrication film, characterized by its low modulus or low surface energy, produces a relatively small shear stress. The formation and performance of the boundary lubrication film are integral to potential-controlled boundary lubrication. This utilization of potential to control boundary lubrication is also referred to as potential-controlled friction. In practical engineering scenarios, mixed lubrication is frequently observed during shifts in operational conditions. This form of lubrication is distinguished by its unique combination of both boundary and fluid lubrication characteristics.
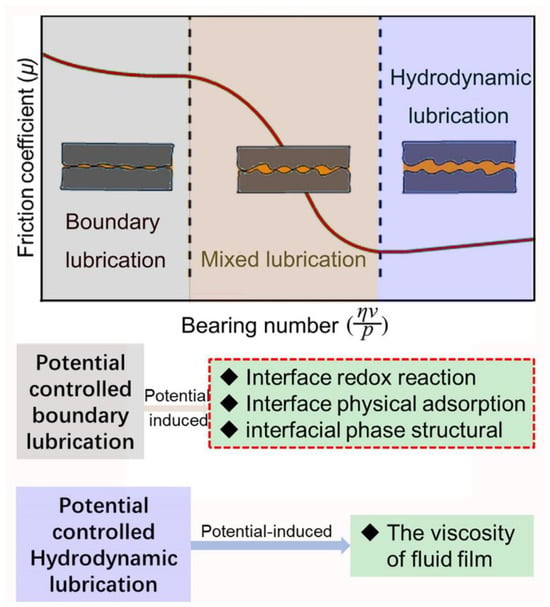
Figure 1. The Stribeck curve delineates three distinct lubrication regimes, with the interface effects of potential significantly influencing both boundary and hydrodynamic lubrication.
2. Two- and Three-Electrode Systems
Potential-controlled friction necessitates the use of potential/electric fields to alter interactions between interfaces. Potential-controlled friction systems that exploit potential/electric fields encompass two-electrode and three-electrode systems. A typical two-electrode friction system is illustrated in Figure 23a, where the entire friction pair is immersed in a solution, with the two friction surfaces acting as the working electrode and the counter electrode, respectively. The frictional performance of the interface is regulated by applying voltage across these surfaces. This control method is characterized by its simplicity and efficiency, particularly demonstrating excellent regulation effects in oil-based lubricants with poor conductivity. This approach has been widely employed by numerous researchers in the early stages of study. In the two-electrode system depicted in Figure 23b, the lower friction pair serves as the working electrode, with the auxiliary electrode acting as the counter electrode. The frictional properties of the interface can be controlled by adjusting the relative voltage between the working and counter electrodes. In this control system, the upper friction pair, which can be composed of non-conductive materials such as ceramics or polymers, does not directly connect with the electrodes. Recent research suggests that integrating this electronic control system with a quartz crystal microbalance offers a powerful tool for investigating the tribotronics of nanoparticle suspensions and ionic liquids [29][39]. Employing this approach to assess the efficacy and responsiveness of a range of materials sensitive to electric fields may fast-track advancements in the realm of electric potential-controlled friction [30][40]. In the control system of Figure 23c, neither the upper nor lower friction pairs serve as electrode materials. The gradient distribution of frictional attributes can be attained by regulating the spatial distribution of interfacial reactions; this regulation is accomplished through intricate adjustments of the current within the solution [31][41]. Despite its simplicity, the two-electrode system has notable shortcomings. Firstly, the precise definition of the true potential on the electrode surface poses a challenge. Even in the absence of applied potential, the electrode potential relative to the standard hydrogen electrode varies amongst different metal electrodes due to their distinct electron gain and loss capabilities. In a two-electrode system, the absence of a reference potential means that the applied potential is merely the voltage difference between the two electrodes, making it difficult to accurately determine the true potential on the electrode surface. Furthermore, the lubricating film situated at the friction pair interface contributes to a voltage drop. As the friction interface undergoes movement, fluctuations in the lubricating film’s thickness cause the interface voltage difference to destabilize, a phenomenon that is challenging to precisely define. This inability to accurately determine the interfacial potential impedes the quantitative study of interfacial reactions. Secondly, the lubricating film between the friction interfaces, typically only several to a dozen nanometers thick, provides limited electrical impedance. A marginally higher voltage can easily breach the lubricating film’s barrier, leading to an interface short circuit. Alternatively, during the friction process, the lubricating film may be compromised by the scraping effect of minuscule peaks, resulting in direct interface contact and the formation of a short circuit. Such short-circuited interfaces disrupt the equilibrium reaction between the original electrode and lubricant molecules, leading to suboptimal potential regulation.
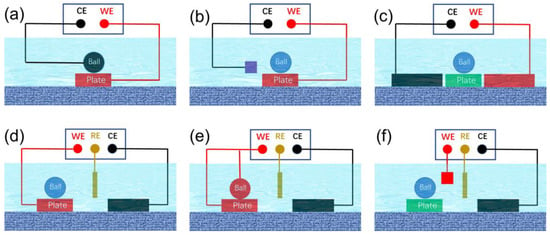
Figure 23. Schematic of a series of electric potential-controlled friction devices (a) A two-electrode control system where the lower friction pair serves as the working electrode while the upper friction pair functions as the counter electrode. (b) In a two-electrode control system where the lower friction pair is employed as the working electrode an auxiliary electrode acts as the counter electrode. (c) A two-electrode control system where the upper and lower friction pairs are not directly linked to the electrode system. (d) A three-electrode control system where the friction pair serves as the working electrode and an auxiliary electrode functions as the counter electrode. (e) A three-electrode control system where both upper and lower friction pairs simultaneously operate as working electrodes. (f) A three-electrode control system in which the upper and lower friction pairs are not directly connected to the electrode system.
In contrast to the two-electrode system, the three-electrode configuration offers superior potential precision and stability, making it particularly advantageous for the design of devices and equipment related to electronic friction control. As depicted in Figure 23d–f, this three-electrode system comprises a working electrode, a reference electrode, and a counter electrode. The current flows between the working and counter electrodes, while the reference electrode maintains a reference potential. The potential at the working electrode’s surface is modulated about this reference potential, thus rendering the surface potential of the working electrode as the actual potential. This system design aids in the precise adjustment of interface potential, thereby facilitating a more accurate study of interfacial triboelectrochemical reactions. In Figure 23d, the lower friction pair serves as the working electrode, while the upper friction pair remains uninvolved in the electrochemical reaction, and can be composed of non-conductive materials such as ceramics or polymers. Interface friction is regulated by controlling the potential at the friction pair’s surface. Figure 23e portrays a scenario where both the upper and lower friction pairs function as working electrodes concurrently, resulting in identical potentials and similar electrochemical reactions on both surfaces. In Figure 23f, neither the upper nor the lower friction pairs are directly connected to any electrode, allowing for the possibility of the friction pair materials being entirely insulating. By fine-tuning the relative position of the friction interface and the electrode system, it is possible to influence the distribution of the boundary lubricating film at the interface. Consequently, even though the frictional pair does not engage in the electrode reaction, this tuning allows its friction performance to be potential-modulated [32][42].
In practical lubrication systems, lubricants adopting hydrocarbon-based ingredients typically exhibit limited conductivity. A more suitable approach appears to involve applying a substantial DC voltage directly onto the friction pair surface using a two-electrode system rather than a three-electrode system. Introducing a supporting electrolyte or leveraging a microelectrode can effectively counteract the poor conductivity of the lubricant, though this strategy is not without drawbacks [33][34][43,44]. The competitive adsorption between the supporting electrolyte and the lubricant additive on the electrode surface may compromise the lubricant’s performance [35][38]. The three-electrode system, especially one based on microelectrodes, imposes stringent environmental stipulations, and the spatial relationship between the friction interface and the electrodes could culminate in uneven lubrication performance at the interface boundary [31][41]. Such conditions constrict the performance tuning capabilities of the three-electrode system within the friction interface of larger equipment. Yet, the precision inherent in the potential of a three-electrode system, along with the convenient quantitative analysis of the electrode process, can prove beneficial for designing compact, high-precision electronic friction transmission systems. Such systems stand to make accurate and swift friction transmission a reality.
