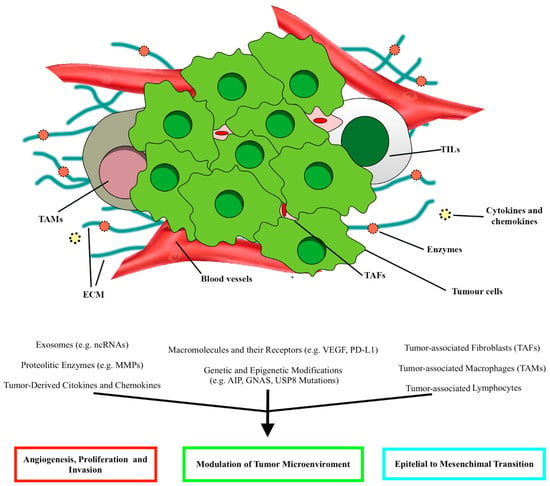
Schematic illustration of the main elements constituting the tumor microenvironment (TME) in PitNETs and the relative effects, according to Marques and Korbonits
. ECM, extracellular matrix; TAFs, tumor-associated fibroblasts; TALs, tumor-associated lymphocytes; TAMs, tumor-associated macrophages.
Macrophages, distinguished in M1 and M2 subpopulations, are immune cells infiltrating mainly sparsely granulated somatotropinomas, null-cell adenomas, and gonadotropinomas.
24.1.3. Stromal Cells
Stromal cells include tumor-associated fibroblasts, myoepithelial cells, and pericytes and can enhance tumor cell proliferation and invasiveness. Three subtypes of tumor-associated fibroblasts have been described: antigen-presenting fibroblasts, inflammatory fibroblasts, and myofibroblasts
[19][23]. Tumor-associated fibroblasts seem to play a crucial role in the progression of PIT1-positive and corticotropic tumors. In invasive PitNETs, fibroblasts are characterized by higher expression of both α-smooth muscle actin (α-SMA) and VEGF, positively influencing the proliferation of GH3 pituitary tumor cells
[21][31].
A potential association between IL-6 and CCL2, secreted by tumor-associated fibroblasts, and high Ki-67 levels was found, speculating how tumor proliferation in PitNETs may be influenced by fibroblasts
[16][22][20,33].
24.1.4. Folliculo-Stellate Cells
Follicle-stellate cells are identified in most PitNETs, and through their ability to release growth factors and cytokines, they maintain a balance between the different cell types and perform immune functions
[23][35]. Recently, an association between increased S100B+ folliculo-stellate cells and lower tumor proliferation was positively related to the expression of estrogen receptor-α and FSH in gonadotropinomas
[24][36]. Higher growth hormone levels were secreted in another study on somatotropinomas with scattered folliculo-stellate cells
[25][37].
24.1.5. Cytokines, Chemokines, and Growth Factors
Cytokines, growth factors, and chemokines, produced by tumor cells and those surrounding the tumor, can trigger cellular defense mechanisms and regulate tumor progression. Cytokines and growth factors, such as CXCL12, CCL5, CCL17, IL-8, IL-6, IL-1, IL-2, IL-17, tumor necrosis factor-α, and vascular endothelial growth factor (VEGF), can affect tumorigenic mechanisms in pituitary neoplasms
[16][20]. Chemokines, such as CXCL8 (or interleukin (IL)-8), CCL2, CCL3, and CCL4, are secreted by pituitary tumors and non-tumor cells, such as macrophages, lymphocytes, or fibroblasts, and modulate the microenvironment composition
[16][20][20,25].
The increased expression of immune checkpoints, such as the programmed death ligand-1 (PD-L1) and the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) ligands CD80 and CD86, has been associated with aggressiveness in proliferative pituitary tumors
[16][20].
The pituitary tumors characterized by elevated expression of PD-L1 presented prevalent immune infiltrates of CD4+, CD8+, and FOXP3+ T cells, highlighting the ability of pituitary tumor-infiltrating immune cells to modulate the expression of immune checkpoint regulators
[16][20]. Moreover, increased expression of the aforementioned CD163 was positively associated with higher expression of PD-L1, PD-L2, and lymphocyte activating 3 (LAG3)
[16][26][20,28]. The increased presence of macrophages, CD4+, CD8+, and CD45+ T cells was related to the expression of V-domain immunoglobulin suppressor of T cell activation (VISTA) and LAG3
[26][28].
24.1.6. The Role of Immunotherapy in Pituitary Tumors: Response and Outcomes
The efforts of the last decades in understanding TME paved the way for new therapeutic options, such as immune checkpoint inhibitors (ICIs). The growing association between the aggressive nature of PitNETs subgroups unresponsive to conventional treatments and the expression of immune checkpoints has increasingly determined the use of drugs targeting CTLA-4 (ipilimumab) or PD-1/PD-L1 (nivolumab or pembrolizumab)
[27][41]. Only a few cases of pituitary tumors treated with ICIs have been reported. Using these drugs in single (such as pembrolizumab) or combined formulations (ipilimumab and nivolumab) allowed a partial radiological response to be obtained in five cases of pituitary carcinoma
[6][28][29][6,42,43]. In comparison, in two other cases, the disease remained stable
[6][28][29][6,42,43]. Maintenance therapy with nivolumab was used in those cases of pituitary carcinoma with an initial response to ipilimumab and nivolumab, showing a radiological response. In the case of disease progression, the carcinoma was retreated with ipilimumab and nivolumab without success, while in the others, a radiological response was documented
[29][43]. Progression-free survival (4 to 42 months) was found in three carcinomas treated with pembrolizumab
[29][43].
The latest efforts aimed at determining which patients could benefit from immunotherapy or how to improve/enhance the therapeutic response for each patient, such as the combined effect of checkpoint inhibitors with peptide receptor radionuclide therapy
[30][44], hypothesize that radiation may influence the TME, further sensitizing the pituitary tumor to the therapeutic response of immunotherapy.
Concerning the individual components of the TME, a tumor-infiltrating immune profile has been associated with poorer outcomes and recurrence in PitNETs
[6]. Moreover, the identification of CD8+ T cells could predict the response to first-generation somatostatin analogs, regardless of tumor characteristics (hormone levels, size, and invasion) and patient age, as demonstrated by Chiloiro et al., where somatotropinomas with high levels of CD8+ and CD138+ lymphocytes responded to first-generation somatostatin analogs
[31][45].
In addition, traditional therapies can influence TME. Somatostatin and its analogs are characterized not only by their anti-tumor action but also by the possibility of influencing the pituitary tumor microenvironment, affecting fibroblasts, and inhibiting the release of cytokines, growth factors, and VEGF from pituitary tumor cells
[16][22][32][33][20,33,50,51]. The immunomodulation of dopamine with DAs, thanks to the presence of receptors on endothelial cells, macrophages, and lymphocytes, has also been described, highlighting the inhibitory effect of cytokine and growth factor secretion, blocking chemotaxis, and inducing apoptosis, especially during the management of prolactinomas and somatotropinomas
[34][35][36][52,53,54].
2.2. PI3K/Akt/mTOR and RAS/MEK/ERK Pathways
4.2. PI3K/Akt/mTOR and RAS/MEK/ERK Pathways
The PI3K/Akt/mTOR and RAS/MEK/ERK pathways are fundamental for cell survival, proliferation, migration, regulation of protein, lipid, and nucleic acid metabolism, senescence, and autophagy. They are triggered by the bond between extracellular ligands and receptor-linked tyrosine kinases (RTK); this bond triggers a cascade of phosphorylation-type reactions that ultimately activate or deactivate various substrates, including transcription factors. This bond, for example, can activate the phosphatidyl-inositol-3-kinase (PI3K), which in turn stimulates the formation of mTOR Complex 1 (mTORC1) and 2 (mTORC2), two protein complexes through which mTOR can operate its effects. Growth factors binding their RTKs also trigger a protein of the Ras family (like H-Ras or K-Ras), which prompts a cascade of sequential phosphorylation-type events that activate RAF, then MEK1/2, then ERK1/2. These kinases interact with various enzymes and transcription factors and also converge on the mTOR pathway. The PI3K/Akt/mTOR and RAF/MEK/ERK pathways are involved in many human neoplasms
[37][38][39][57,58,59].
EGFR signaling, which involves the mTOR pathway, is expressed in these tumors, and it is associated with tumor proliferation, invasive behavior, lower total resection, and epithelial-to-mesenchymal transition
[40][41][42][60,61,62]; this latter is also mediated by the ADAM12 metalloprotease
[42][62]. IGF-1 signaling also exerts a mitogenic effect through the PI3K/mTOR/Akt pathway
[43][44][63,64].
Expressing platelet-derived growth factor (PDGF) and its receptor by folliculostellate cells stimulates pituitary cell proliferation through the PI3K/mTOR/Akt pathway
[45][65].
Mutation of the proto-oncogene PI3KCA (which encodes a subunit of PI3K) was found in 2.3% to 12.1% of tumor series, while amplification of the same gene was documented in 21.2% to 28% of cases
[46][47][66,67]. Mutations of PTEN do not seem to occur frequently
[48][68], but it is downregulated in PitNETs
[49][69]. In its phosphorylated form, Akt is more expressed in PitNETs than in normal pituitary tissue
[48][50][51][52][53][54][55][68,70,71,72,73,74,75], especially in recurrent tumors
[51][71].
The mTOR molecule was shown to be expressed in PitNET cells
[56][76] alongside two mTOR-related proteins, RAPTOR (part of mTORC1) and RICTOR (part of mTORC2)
[49][69], with RAPTOR expression being associated with CS invasion. Unlike normal pituitary tissue, downstream effectors of mTOR, like phospho-S6 protein and phospho-4EBP1 protein, are increased in PitNETs
[54][57][74,77]. DEPTOR, a down-regulator of mTOR, is underexpressed in PitNETs
[58][78].
The RAF/RAS/MEK/ERK pathway, and especially the ERK molecule, is also overexpressed in PitNETs
[42][43][50][51][56][57][59][60][61][62][62,63,70,71,76,77,79,80,81,82] and was shown to be regulated by the PI3K/Akt/mTOR pathway
[62][82]. ERK also mediates the signaling of different growth factors, like EGFR
[40][60] and IGF-1
[44][64], in pituitary tissue. The transcript of BRAF is overexpressed in pituitary adenomas compared to normal pituitary
[63][83], and the BRAFV600E mutation was found in 16.5% of corticotroph adenomas
[64][84].
In summary, current evidence points to overexpression and hyperactivation of crucial molecules of the PI3K/Akt/mTOR and RAF/MEK/ERK pathways in PitNETs.
Rapamycin (also known as Sirolimus) and its analog Everolimus (also known as RAD001) inhibit mTOR directly by binding the FKBP12 protein, forming a complex that interacts with the mTOR molecule and prevents it from forming mTORC1 and mTORC2. These drugs can reduce the number of viable PitNET cells, their proliferation, and the phosphorylation of downstream mTOR effectors
[53][55][65][66][67][73,75,86,87,88], lower prolactin secretion, decrease mTOR phosphorylation, enhance the radiotherapy response, and block IGF-I proliferative and anti-apoptotic effects
[65][67][86,88]. The anti-proliferative effects of Everolimus are enhanced by the co-treatment with Pasireotide
[67][88], and a similar effect was observed for Rapamycin with Octreotide
[68][89].
Inhibitors of PI3K, like the pan-PI3K inhibitor NVP-BKM120 (Buparlisib) and the specific PI3K-alpha inhibitor NVP-BYL719 (Alpelisib), have shown a dose-dependent inhibition of cell viability of PitNETs and display a synergistic effect when combined with Everolimus
[66][87]. Inhibition of PI3K with the chemical compound LY294002 reduces PitNET cell growth, increases the pro-apoptotic activity of Bcl2-associated death promoter, decreases the anti-apoptotic effect of IGF-1, and decreases phosphorylation of PI3K and Akt
[44][54][69][70][71][64,74,91,92,93].
Dual PI3K-mTOR inhibitors like NVP-BEZ235 (Dactolisib) seem more effective than Everolimus in reducing the cell viability of PitNETs
[72][94]. NVP-BEZ235 treatment decreases Akt and S6 phosphorylation and triggers apoptosis
[72][73][94,95]. Another dual inhibitor, XL765 (Voxtalisib), enhances the effects of temozolomide against PitNET cells
[73][95].
Gefitinib, an anti-EGFR tyrosine kinase inhibitor, reverses the epithelial-to-mesenchymal phenotype, decreases invasiveness, and reduces the proliferation of PitNETs
[42][62]. In another study, Gefitinib resulted in tumor shrinkage and a reduction in peripheral hormone levels by around 30% in a mouse model. Gefitinib treatment in mice decreased ERK1/2 phosphorylation, followed by downregulation of tumor prolactin mRNA
[40][60].
Among other lesser-known drugs, the Akt inhibitor MK-2206 was shown to reduce the phosphorylation of Akt. The HIV protease inhibitor Nelfinavir radiosensitizes PA cell lines in vitro, and the underlying mechanism seems to involve the mTOR pathway
[74][96].
Corticocotroph PitNET cells harboring the BRAFV600E mutation undergo a more significant reduction in hormone secretion when treated with BRAF inhibitor Vemurafenib, compared to tumor cells with wild-type BRAF
[64][84].
In summary, drugs targeting the PI3K/Akt/mTOR pathway seem capable of interfering with PitNET growth, survival, and hormone secretion and enhancing the effects of other therapeutic strategies like radiotherapy, somatostatin analogs, and temozolomide. Still, clinical studies are required to study their effectiveness further.
2.3. Receptors
4.3. Receptors
24.3.1. Somatostatin and Dopamine Receptors
Somatostatin receptors (SSTR) and dopamine receptors (DRD) represent a staple in PitNETs therapy, and their agonists are commonly used in clinical practice, especially in PIT-1-positive tumors (GH and PRL-positive subtypes), except corticotrophs and gonadotrophs. In particular, in Cushing’s patients, SSTR5, SSTR2, and SSTR3 are predominantly expressed, representing three of the four target receptors of pasireotide
[75][76][97,98].
A novel pathway has been described by Peverelli et al. that might explain SSTR2 anti-neoplastic activity and its role in PitNETs pathophysiology via the activation of the Rhoa/ROCK pathway and the consequent cofilin phosphorylation; this way, cofilin is unable to bind to actin, and SSTR2 activation inhibits cytoskeleton remodeling and cell migration
[77][103]. The exact mechanism for the DRD2 isoform was described by Peverelli et al., who also correlated low cofilin phosphorylation with invasion in aggressive PitNETs
[78][104]. New molecules have been proposed, such as BIM23120 (a selective SSTR2 agonist) and BIM53097 (a selective DRD2 agonist), showing promising results in reducing somatotroph cell migration and proliferation and inducing apoptosis.
SSTR5 is highly expressed in NF-, ACTH-, and GH-PitNETs and correlates with recurrence
[76][98]. When analyzed in association with DRDs, it has been shown to form chimeric receptors, particularly with DRD2
[79][80][108,109], and the chimeric receptor SSTR5/DRD2 has been linked with inferior dimension and grade
[80][109]. Its targeting via BIM23A760 (a selective agonist) reduced GH and PRL production in vitro; in GH tumors, it reduced cell viability and proliferation and stimulated apoptosis
[79][108].
Other SSTRs were less investigated, given their lower representation. The data above have revived interest in these receptor subtypes, and there is evidence that SSTR1 might represent an indicator for response to medical treatment and a prognostic factor: its overexpression has been described in recurrent ACTH-secreting tumors
[76][98], and low levels are linked with remission after first surgery
[81][107].
As for SSTR3, Lee et al. suggested its anti-neoplastic role is due to its implication in the MAPK pathway: SSTR3 activated tyr-phosphatases, ultimately inhibiting MAPK and consequently activating p53 and the caspases, promoting apoptosis
[82][110]. SSTR3 is highly expressed in SF-1 positive PitNETs, even recurring ones
[82][110], while it is reduced in recurring NF PitNETs
[76][98].
24.3.2. Peptide Receptor Radionuclide Therapy
Peptide receptor radionuclide therapy (PRRT) is an innovative therapeutic option traditionally used as a second-line treatment for advanced (metastatic or inoperable) neuroendocrine tumors (NETs), characterized by the expression of the SSTRs, especially the SSTR2
[83][84][111,112]. The applicability of such treatment is closely related to the expression of somatostatin receptors, which can be evaluated through functional imaging such as 68Ga-DOTA peptide PET/CT, octreoscan, 111In-octreotide-scintigraphy, or 99m-EDDA-HYNIC-tyr3-octreotide scintigraphy
[10][85][86][87][88][89][90][91][92][93][94][95][96][10,113,114,115,116,117,118,119,120,121,122,123,124].
24.3.3. Transforming Growth Factor Receptor
Transforming growth factor beta (TGF-β) signaling is related to numerous biological processes involved with cell proliferation, differentiation, apoptosis, and EMT. Its action is mediated by TGF-β receptor complexes (TGF-β RI and RII) that phosphorylate Smad2 and Smad3, which in turn form trimers with Smad4 and translocate to the nucleus, regulating the expression of genes controlling the cell cycle
[97][126]. TGF-β has been investigated in different types of cancer, and it can act as either a suppressor or an inhibitor of tumor development, depending on the tumor and stage
[98][127]. Given its intricate relationship with tumorigenesis, new data are being implemented to uncover its role in PitNETs.
Some authors have described that TGF-β is elevated in aggressive PRL-secreting tumors, both locally
[99][128] and in the serum (and serum TGF-β is directly correlated with tumor dimensions and aggressivity)
[100][129]. Jiang et al. correlated TGF-β levels with microvascular density in the neoplastic tissue
[99][128].
On the contrary, when analyzing the TGF-β pathway, Ying-Hao et al. reported an inverse association between TGF-β RII expression and invasiveness, thus documenting it as a tumor suppressor. No significant difference was highlighted for TGF-β RI
[101][133].
24.3.4. Fibroblast Growth Factor Receptors
Fibroblast growth factor receptors (FGFR) are tyrosin-kinase receptors, and their activation mediates cell proliferation, migration, and apoptosis
[102][136]. The involved pathways include MAPK and phosphatidylinositol-3-kinase. Ptd-FGFR4 is the N-terminally truncated isoform of FGFR4, characterized by cytoplasmic localization and constitutive phosphorylation
[103][137]. Analyzed against common biomarkers of aggression and invasiveness, ptd-FGFR4 showed a direct correlation with Ki-67
[104][138]. Accordingly, Brito et al. documented that all patients with recurrent Cushing’s disease had high levels of FGFR4 mRNA expression
[105][139].
24.3.5. Folate Receptor
Folate receptors (FR) are glycosylphosphatidylinositol-anchored membrane proteins involved in the absorption of folic acid, essential for cell proliferation. Among the three human isoforms (α, β, and γ), FRα is overexpressed in some forms of cancer, such as ovarian and cervical carcinomas, and, although less commonly, in lung and breast cancers. At the same time, it is poorly expressed in normal tissues
[106][142]. FRα is strongly upregulated in NF-PitNETs but is absent or downregulated in functioning PitNETs or normal pituitary glands
[107][108][109][110][143,144,145,146].
In a clinical study on 56 NF-PitNETs, a pre-operative SPECT/CT image after 99mTc-EC20-folate administration documented a sensitivity of 81% and a specificity of 83%, highlighting the possibility of an appropriate selection of patients who could take advantage of this treatment
[111][150].
24.3.6. Estrogen Modulators
The relationship between estrogen hormones (ES) and their receptors (ESR) plays a crucial role in the pathogenesis of PitNETs. In normal human tissue, ERα (also called ER1, ESR1, and NR3A1) and Erβ (known as ER2, ESR2, and NR3A2) are nuclear receptor isoforms that respond to 17β-estradiol (E2), leading to cell proliferation and differentiation, respectively
[112][113][152,153]. Among the best-known ERα receptors, ERα66 and its variant ERα36, which are situated in both the cytoplasm and plasma membrane, are mostly found
[114][154].
Studies performed on lactotroph pituitary adenomas highlighted aggressive behavior, especially in the male gender, when the expression of ERα was reduced
[115][116][117][118][119][120][121][158,159,160,161,162,163,164], probably associated also with the expression of genes situated on chromosome X (CTAG2, FGF13, and VEGF) that influence the ER pathway
[115][158]. Recently, Mahboobifard et al. found a decreased expression of ERα66 and ERα36 in PRL-PitNETs compared with normal pituitary tissue. Furthermore, invasiveness was associated with low levels of ERα36 and ERα66, while an increased Ki67 index was related to decreased ERα36 expression. A significant inverse association between ERα66 with dopamine-agonist resistance and tumor size was also documented
[122][165].
The association between ER and invasiveness is still debated
[115][123][124][158,171,172]: Zhou et al. found a significantly higher expression of nuclear ERα staining in invasive NF-PitNETs (especially in females) than non-invasive ones. In contrast, ERβ staining decreased in invasive NF-PitNETs. Other studies found lower ERα mRNA levels in non-invasive prolactinomas but also significantly lower levels of ERα in invasive pituitary tumors
[119][121][122][125][162,164,165,167].
24.3.7. Wnt/β-Catenin and E-Cadherin
The Wnt/β-catenin pathway, also known as the “canonical” Wnt pathway, has been linked to many human diseases, cancerous and non-cancerous
[126][183]. Once activated by the canonical pathway, β-catenin translocates into the nucleus. It induces genes involved with cell proliferation and migration (e.g., c-myc, MMP)
[126][183].
Moreover, β-catenin intervenes in cell-cell adhesion by forming a heterodimer with E-cadherin (which, in turn, inactivates the mitogenic function of β-catenin when complexed together); the E-cadherin/β-catenin anchoring complex is known to maintain epithelial cell differentiation and adhesion, and its dysregulation has been recognized as a promoter of epithelial–mesenchymal transition in many cancers
[127][184].
The evidence suggests pituitary cells lose their normal differentiation and transition to a tumorous phenotype when membranous E-cadherin is downregulated. However, therapeutic options are not available yet. E-cadherin might represent a molecular therapy target in the future.
B-catenin shows a multimodal pattern: when analyzed alone, nuclear accumulation of B-catenin positively correlates with PitNETs aggressivity
[128][129][192,193]. On the other hand, when E-cadherin is considered, B-catenin downregulation is linked to aggressive behavior and recurrence
[130][191], further suggesting that E-cadherin serves as a proto-oncogene in PitNETs. Temozolomide has effectively reduced B-catenin activation and nuclear translocation, impairing cells vitality and promoting their apoptosis. It also reduced prolactin production in PRL-secreting adenomas in animal models
[131][194].
24.3.8. Galectin-3
Galectin-3 (Gal-3) is a β-galactoside-binding lectin expressed in various types of cancers and plays a vital role in PitNET cell proliferation
[132][133][195,196]. An increased Gal-3 expression in PRL− and ACTH-PitNETs has been described, but why and how it is involved in pituitary tumor progression is still unclear. Gal-3 levels further increase in the progression from PRL and ACTH-secreting adenomas to carcinomas; gene methylation plays a role in Gal-3 expression, and RUNX1 and RUNX2 transcription factors seem to target its gene directly, enhancing its expression
[132][195].
2.4. Matrix MetalloProteinases
4.4. Matrix MetalloProteinases
MMPs are zinc-containing calcium-dependent endopeptidases paramount in extracellular matrix (ECM) degradation and remodeling by acting on several substrates. The increasing evidence of the importance of ECM remodeling in tumor invasion supports the relevance of those enzymes in promoting PitNET local invasiveness.
MMP-9 has been widely studied as it acts by notably degrading type IV collagen, which represents the main component of the basal membrane and the medial wall of the CS. In 1996, for the first time, Kawamoto et al. observed MMP-9 expression to be significantly increased in invasive PitNETs when compared to non-invasive ones
[134][200].
Moreover, they also found that the expression of MMP-9 was strongly associated with the recurrence-free interval, suggesting that patients with high MMP-9 expression may need particularly close clinical and radiologic follow-up after surgery
[135][205]. Gong et al. found increased MMP-9 mRNA expression in the invasive PitNETs
[136][206]. However, several studies also showed no correlation between MMP-2 and MMP-9 expression, as previously reported by other groups
[137][138][207,208].
Protein kinase C (PKC) is a ubiquitous family of enzymes involved in several cellular pathways, such as mediating cell growth and tumor invasion by activating MMP-9. Hussaini et al. demonstrated increased expression and activity of MMP-9 in invasive NF-PitNETs and PKC-activated HP75 cell lines
[137][207]. PKC activated MMP-9 in a highly cell-type-specific manner. Moreover, some studies found that invasive PitNETs were characterized by point mutations of PKC-α and higher overall PKC activity and expression
[139][140][209,210]. The effect of several biological agents on MMP-9 activity and expression by either positively or negatively influencing PKC overall activity has been tested in different tumor models.
2.5. Angiogenesis
4.5. Angiogenesis
24.5.1. Vascular Endothelial Growth Factor
Vascular endothelial growth factor (VEGF), especially the subtype VEGF-A, represents a fundamental mediator of vasculogenesis, angiogenesis, vascular permeability, cell survival, and migration
[141][227]. The expression of VEGF is regulated by several factors, such as hypoxia-inducible factor (HIF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF). In tumorigenesis, the upregulation of VEGF and its receptors is commonly found in most tumors, impacting their invasiveness, the formation of metastasis, and cell survival through the induction of bcl-2 expression
[141][142][227,228]. Therefore, anti-VEGF therapy has been progressively introduced and approved as a secondary-line therapy
[143][229]. The available data on VEGF expression for managing aggressive PitNETs are conflicting. Some authors identified overexpression of mRNA VEGF in invasive tumors and carcinomas, especially in the elderly, and NF-PitNETs. At the same time, other studies did not find a significant correlation between VEGF expression and tumor behavior
[117][144][145][146][147][160,221,230,231,232].
The results from in vitro experiments have shown that the anti-VEGF-A mAb G6-31 inhibited tumor growth and decreased serum prolactin levels, especially in dopamine-agonist-resistant prolactinomas
[148][235]. Zhou et al. experimented with Cabozantinib, an inhibitor of VEGFR2, on RBΔ19 mice, prolonging mean survival in a dose-dependent manner (15 mg/kg and 30 mg/kg)
[149][236]. On prolactinomas, Axitinib, a VEGFR-selective tyrosine kinase inhibitor, stopped tumor progression, decreased vascular density, and, through the combination of bromocriptine, reduced tumor bleeding and normalized the vessel architecture
[150][55].
24.5.2. Endocan
Endocan, also known as endothelial cell-specific molecule-1 or ESM-1, is a soluble chondroitin/dermatan sulfate marker of neoangiogenesis closely related to VEGF and FGF expression. Its role in physiological processes is well known and has an essential effect on inflammation and tumor pathogenesis
[151][240]. In the normal pituitary gland, only a few endocrine cells expressed endocan, while in pituitary tumors, the data about its expression are inconsistent
[152][153][154][155][219,241,242,243].
Even today, the role of Endocan in target therapy is still to be defined, especially for pituitary pathology. Studies on other tumors have shown that it is possible to block endocan expression by acting on the VEGF pathway through tyrosine kinase inhibitors or VEGF antibodies
[156][245]. Recently, monoclonal antibodies, direct synthetic peptides, and endocan silencing have been developed, promoting apoptosis, tumor volume reduction, and cell migration in several tumors
[157][158][246,247].
2.6. Genetic Aspects in Aggressive PitNETs
4.6. Genetic Aspects in Aggressive PitNETs
24.6.1. Germline Mutations
Pituitary tumorigenesis may also be caused by germline mutations that are part of a syndromic presentation or in isolation. The first identified gene underlying familial isolated pituitary adenoma (FIPA) is AIP (Aryl hydrocarbon receptor interacting protein), which accounts for about 10–20% of FIPA kindreds
[159][249]. It encodes a co-chaperone protein, which acts as a tumor suppressor, especially versus GH and PRL PitNETs. The prevalence of AIP mutations in patients with sporadic pituitary tumors is exceedingly rare, and it is higher among acromegalic patients and patients with less than 30 years of age at diagnosis
[160][250]. Some data suggest that low AIP expression is a better marker of invasiveness in somatotropinomas than the Ki-67 labeling index and p53
[161][251].
24.6.2. Somatic Mutations
Despite applying novel exome and whole genome sequencing techniques in the study of PitNETs, a few recurrent mutated genes have been reported
[162][163][256,257]. Efforts in this respect will be paramount in developing novel therapeutic strategies and additional treatments for this kind of tumor. No apparent recurrent driver alterations have been identified for PitNETs, apart from GNAS and USP8 mutations in GH− and ACTH-PitNETs, respectively.
GNAS
GNAS is a complex imprinted gene characterized by several promoters. One of its products, the stimulatory α subunit of G-protein, is paramount in regulating the enzymatic activity of adenylyl cyclase. Tumor cell proliferation in PIT1 might be associated with GNAS copy number gain
[164][258]. At the same time, mutations affecting GNAS codons 201 or 227 may lead to prolonged GTPase activity and increased cAMP levels, playing a role in GH secretion in acromegalic patients
[165][259].
Increasing evidence suggests that GNAS-mutated tumors are more likely to be smaller, non-invasive, and better respond to SSAs
[165][166][167][168][259,260,261,262], despite some authors showing no differences in tumor extension or response to SSAs between mutated and non-mutated tumors
[169][263].
USP8, also known as ubiquitin-specific protease Y (UBPY), is a deubiquitinating enzyme displaying elevated activity toward EGFR and other receptor tyrosine kinases (RTKs) with similar signaling cascades
[170][171][264,265]. USP8 gain-of-function mutations protected EGFR from lysosomal degradation, thereby increasing its expression. Interestingly, USP8 mutations have been only documented in sporadic Cushing’s disease. The most common somatic mutation involved exon 14, which encoded a putative binding domain
[172][266].
USP8-mutated tumors are associated with an earlier onset and a higher risk of recurrence
[173][267]. Retrospectively analyzing their cohort of patients, Albani et al. reported that the incidence of relapse over a 10-year follow-up in patients who obtained biochemical remission after surgery was higher in patients with tumors with the USP8 mutation
[174][268]. In another cohort of pediatric patients with Cushing’s disease, recurrences have been documented in tumors with USP8 mutations
[175][269].
2.7. Future Perspectives in PitNETs: The Role of Epigenetic
4.7. Future Perspectives in PitNETs: The Role of Epigenetic
24.7.1. DNA Methylation
The development of new DNA methylation detection and analysis techniques has provided novel information on the pathogenesis of pituitary tumors. In 2012, the first genome-wide analysis of the DNA methylome in PitNETs examined more than 14,000 genes
[176][273]. Since its early applications, methylome profiling has appeared helpful in understanding PitNET pathophysiology. However, up to date, global DNA methylation profiling has failed to predict clinically significant differences among pituitary tumors
[177][274].
DNA methylation is crucial in PitNETs pathophysiology, mainly driving the loss or reduced expression of tumor suppressor genes (TSGs)
[178][275]. Many of those genes have been shown to harbor C-phosphate-G (CpG) island hypermethylation, determining their silencing. Ling et al. identified 34 CpGs on 17 genes, characterized as being hypomethylated in invasive NF-PitNETs compared to non-invasive ones. Interestingly, among differentially methylated genes FLT1 (Fms-related receptor tyrosine kinase 1) and SLIT3 (slit guidance ligand 3), most of them were associated with invasion and cell migration
[179][276].
Interestingly, a single miR may have different target genes. Duan et al. showed that the ectopic expression of miR-137, which is reduced in pituitary tumor tissues compared to normal controls, interfered with the proliferation and invasion of pituitary tumor cells via targeting AKT2 (AKT serine/threonine kinase 2)
[180][290]. Moreover, Wei et al. reported miR-137 to target EGFR, thus inhibiting cell proliferation of GH3 cells and inducing apoptosis and G1-phase arrest. According to the authors, miR-137 mimic and AZD9291 (Osimertinib, an oral third-generation EGFR tyrosine kinase inhibitor)-mediated additive effects in GH3 cells
[41][61].
2.8. Molecular Target Therapy and Clinical Prospects
4.8. Molecular Target Therapy and Clinical Prospects
Molecular assessments, such as those described in the present work, could represent the key factor for the treatment of those subtypes of aggressive PitNETs that are not responsive to conventional therapies. In this view, the 2017 and 2022 WHO classifications of endocrine tumors tried to define and identify this subgroup of neoplasia
[181][182][294,295]. In particular, the 2017 WHO classifications defined as “high-risk categories“ prolactinomas in males, poorly granulated somatotrophs, silent corticotrophs, Crooke cells, PIT1-positive silent plurihormonal PitNETs, and high Ki67% index PitNETs
[181][294]. The recent update of the 2022 WHO PitNETs classification added the role of tumor cell lineages, distinguishing between immature and mature tumors of the PIT1 lineage and defining the inclusion features of the PIT1 lineage “family”
[182][295].