Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Tsvetelina Velikova.
The gastrointestinal (GI) tract may be a significant entrance or interaction site for SARS-CoV-2; therefore, the gut mucosal immune system participates in virus interaction as a first-line physical and immunological defense, leading to GI involvement and symptoms. The pattern of symptoms changed during the virus evolution, since the data provided a current and thorough picture of the symptoms experienced by SARS-CoV-2 infected people, and variations in symptom patterns occurred as the Alpha, Delta, and Omicron variants have spread. Since the beginning of the pandemic, GI symptoms have been linked to SARS-CoV-2 infections, even though most infected people do not report them.
- COVID-19
- SARS-CoV-2
- gastrointestinal symptoms
- diarrhea
1. Introduction
In late November 2021, South Africa reported the latest variant B.1.1.529 of severe acute respiratory syndrome virus 2 (SARS-CoV-2) to the World Health Organization (WHO). Compared to the other SARS-CoV-2 variants, the new variant has a higher risk of infection, spreads faster, and has been named “Omicron” by the WHO [1]. Since then, the omicron variant BA.1 has spread rapidly worldwide, displacing the previously dominant delta variant. In addition, other variants evolved and spread: Omicron BA.2, which has 1.4 times the effective reproduction number of the mutant BA.1, with vaccine-induced immunity being primarily ineffective, and then two other sub-variants, BA.4 and BA.5, replaced BA.2 as the dominant strains in South Africa in early May 2022, and their transmission ability increased [1,2,3][1][2][3].
Menni et al. demonstrated that other organ complications, such as gastrointestinal problems (GI), were uncommon in the last virus variants. However, because the GI tract may be a significant entrance or interaction site for SARS-CoV-2, the gut mucosal immune system participates in virus interaction as a first-line physical and immunological defense [2]. Additionally, GI involvement and symptoms have been associated with poorer clinical outcomes in COVID-19 patients [4].
A prominent feature of SARS-CoV-2 is that it is a mucosal pathogen that infects human respiratory epithelial cells and the GI tract by latching to angiotensin-converting enzyme 2 (ACE-2) receptors through the spike (S) receptor-binding domain. Therefore, mucosal immunity is essential for sufficient and long-term viral protection [5].
Moreover, the GI system is a common entry point for SARS-CoV-2. After exposure, the virus disturbs the mucosal immune system, but further studies are needed to understand all the intimate immunological mechanisms and processes in the gut mucosa, where the recruitment of immune cells such as neutrophils, dendritic cells, macrophages, and T cells are involved in the immune responses. The mucosal inflammatory response, on the other hand, may alter the intercellular space between enterocytes, increasing intestinal permeability and allowing numerous bacterial antigens and toxins to enter the bloodstream, aggravating COVID-19 [6].
2. COVID-19, Mucosal Involvement and GI Symptoms
Typical clinical symptoms of fever, dry cough, fatigue, sputum production, and dyspnea accompany COVID-19. Although the main symptoms that characterize the disease are respiratory, other extrapulmonary manifestations, such as GI, neurological, renal, hepatic, cardiovascular, endocrine, dermatological, etc., have been reported [16][7]. Among the GI symptoms, anorexia (loss of appetite), nausea, vomiting, diarrhea, and abdominal pain were the most common. Less observed but more severe were mesenteric ischemia and GI bleeding. It should be mentioned that GI symptoms complicate the disease, increase the duration of the illness, and result in worse outcomes [17][8]. As mentioned above, the entrance of SARS-CoV-2 in the host cells has been described in two ways. The first way is by ACE-2 receptor-mediated endocytosis, and the second is by ACE-2 receptor plus transmembrane peptidase/serine subfamily member 2/4 (TMPRSS2/4) membrane fusion [18][9]. The ACE-2 receptors are abundant in most GI organs [19][10]. Their primary function is converting Angiotensin I to Angiotensin (1–9) and Angiotensin II to Angiotensin (1–7), promoting anti-inflammation [20][11]. Moreover, ACE2 receptors are responsible for the uptake of dietary amino acids, thus regulating antimicrobial peptides and preserving a healthy GI microbiome [22][12]. Host infections usually lead to the downregulation of the ACE-2 receptor activity. Therefore, direct inflammatory damage to enterocytes and a change in the normal function of the intestinal microbiome could be observed [23,24][13][14]. The disbalance of the gut microbiome leads to further inflammation and a series of immunological processes that are not limited to the GI system but also affect the respiratory tract [25][15]. The GI and respiratory systems have their own microbiomes and might be linked through immunological processes in the mucosal surfaces [26][16]. This link is usually mentioned in the literature as the gut–lung axis, and it has shown a two-way contribution in developing diseases in both systems. Thus, enhancement of the gut microbiome with probiotics has a possible positive effect on preventing and treating COVID-19 [27][17]. In COVID-19, anorexia, loss of taste, fever, and sedation contribute to reducing food intake, malnutrition, muscle wasting, and sarcopenia [28][18]. While anorexia commonly follows infections, it can also result from nausea [29][19]. Furthermore, anorexia, nausea, and vomiting have mechanisms that include the area postrema. More specifically, for nausea and vomiting, two mechanisms were proposed. One is by activating vagal afferents, and one is by releasing neuroactive agents that act in the area postrema. Both mechanisms activate the nucleus tractus solitarius and cause the induction of nausea and vomiting [30][20]. Diarrhea could be caused by the direct virus actions on the host or by iatrogenic factors, such as medications. In each case, the progress of the disease is complicated, the microbiome is damaged, and inflammation is promoted [31][21].
Additionally, GI involvement may attenuate the immune response and decrease the risk of cytokine storm and systemic inflammation, leading to better patient outcomes and prognosis. Other investigators, such as Megyeri et al., extensively researched COVID-19-associated diarrhea, focusing on the following mechanisms: direct gut epithelium damage, gut microbiota alteration, and mucosal inflammation. However, they concluded that these changes may worsen outcomes and exacerbate the symptoms [33][22].
The characteristics of the COVID-19 patient with pain in the upper abdomen are prominent dyspnea, increased admission to ICU, and poor outcomes. In contrast, lower abdomen pain has less noticeable dyspnea, fewer admissions to ICU, and better results [34][23]. In non-COVID patients, the primary etiology of mesenteric ischemia is a mesenteric arterial embolism, mesenteric arterial thrombosis, venous thrombosis, or, less frequently, low-flow non-occlusive causes. While the pathophysiology of these alterations for the COVID-19 group is unclear and the prevalence is low, the reasons should closely mirror the non-COVID patients and be classified again as occlusive and non-occlusive. Emergent management must be performed if mesenteric ischemia is diagnosed [35][24].
In summary, early reports of COVID-19 GI involvement included nausea, vomiting, diarrhea, and loss of appetite in 32% of patients, sometimes preceding the respiratory symptoms [37][25]. Then, recent reports declared GI symptoms were prevalent, with up to one-third of COVID-19 patients presenting with GI symptoms first. Moreover, nausea and vomiting may occur in up to two-thirds of people with COVID-19. Around 40% of COVID-19 patients may experience appetite loss, and up to 50% would experience diarrhea. Abdominal discomfort was less prevalent, affecting fewer than 10% of people [38][26]. Nevertheless, it is accepted that the Omicron variant causes milder COVID-19 than the Delta [39][27].
3. Virus Variants Associated with Gastroenterology Syndromes
According to different data, GI involvement in SARS-CoV-2 infection has been underestimated, ranging from 3% to 79%. As stated above, over 45% of patients have diarrhea as a primary complaint [44][28]. An interesting observation was made by Natarajan et al. that, even after as long as 4 months after infection, viral shedding and detectable viral SARS-CoV-2 RNA were found in fecal samples of COVID-19 patients with GI symptoms [45][29]. That correlated with the observations made early in the pandemic. Due to the high expression of the ACE 2 receptor in differentiated enterocytes, researchers made an experimental model that proved the possibility of viral replication in the enterocytes in the small intestine and the production of infectious viral particles [47][30]. Still, there is no answer as to whether the GI symptoms most patients experienced were a result of an immune response or the direct cytopathic effects of the virus. A plausible explanation was given by Lehmann et al. [48][31], proving the activation and recruitment of cytotoxic T lymphocytes in the intestinal mucosa. The high expression of TMPRSS2 in the mucosa of the small intestine and the even higher ACE2 expression in the gut encouraged the team to perform duodenal biopsies via esophagogastroduodenoscopy. Although there were no prominent macroscopic findings during the procedure, the histopathologic analysis revealed that CD8+ cell infiltration was an obvious sign of a local immune reaction. All of the above explains commonly observed symptoms during SARS-CoV-2 infection, such as diarrhea, nausea and vomiting, anorexia, and abdominal pain in 11.51% of all COVID-19 patients in the metanalysis by Merola et al. [49][32]. As previously reported, SARS-CoV-2 fecal shedding was found in 40.5% of confirmed cases in another paper [50][33]. SARS-CoV-2 is a virus that rapidly evolved, leading to changes in the symptoms experienced by infected individuals. Therefore, Schulze et Bayer discussed the differences in signs from the first wave (Alpha) to the last waves (Omicron) regarding the symptomatic infection and symptoms from respiratory, GI, neurology, etc., and the symptoms changed evenly over time [54,55][34][35]. The widely accepted respiratory symptoms include those resembling the common cold, loss of sense of smell and/or taste (confirmed by multiple meta-analyses), fever, fatigue, headache, muscle and joint pain, sore throat, etc. The pattern of symptoms described by SARS-CoV-2-positive people varied dramatically over time, with nasal symptoms becoming more common since the development of the Alpha and Delta variants and the characteristic loss of smell and taste becoming substantially less common after the advent of the Omicron variant [54][34]. In addition, GI symptoms have been linked to SARS-CoV-2 infections since the outset, even though most infected people do not report them. Diarrhea (28.2%) was the most frequently reported GI symptom in the early phase of the pandemic (G614-dominated), but its rate has declined in the following variant-dominated phases (Alpha: 19.4%, Delta: 17.9%, Omicron: 13.8%) [54][34].4. Specific Digestive System Involvement in COVID-19
4.1. Pancreatic Involvement
Although SARS-CoV-2 infection was previously thought to mainly attack the pulmonary system, recent studies imply that the pancreas may be another potential target for the infection. There has been proof that, after infection, patients are at increased risk of developing diabetes mellitus. The reason behind that is the high concentration of ACE2 receptors and TMPRSS2 expression in the pancreas, especially in the Langhans islets beta cells and the pancreatic blood vessels [58][36]. Both exocrine and endocrine functions of the pancreas can be involved. However, a rare complication of acute pancreatitis with epigastric pain and highly elevated amylase and lipase can be one of the many presentations of coronaviral infection. Elevated pancreatic serum markers can result from multiorgan dysfunction, in severe cases, accompanied by a cytokine storm. Newly onset metabolic disorders, including hyperglycemia, are present in COVID-19 patients [58,59][36][37]. Patients often develop the so-called Post-Acute Sequelae of CoV-2 (PACS) after infection. Scherer et al. focused on the metabolic changes following SARS-CoV-2 infection. Beta-cell dysfunction, insulin resistance, and the failure of the liver to produce glucose, as well as insufficient pancreatic insulin production and chronic inflammation, are among the main reasons for postinfectious complications, which is a consecutive proof of pancreatic, liver, and GI involvement in the pathogenesis of the virus and its sequelae [60][38]. An updated systematic review and meta-analysis by Lee et al. [62][39] reported the susceptibility and clinical outcomes in IBD patients infected with SARS-CoV-2. IBD conditions were not associated with an increased risk of hospital or ICU admission or death, although 5-ASA and corticosteroids were associated with an increased risk for these events. From the gastroenterologist’s perspective, underlying conditions, such as IBD or chronic liver disease, and immunosuppressive therapy may impact the risk of developing GI complications related to SARS-CoV-2 infection and their diagnosis and treatment [63][40]. Bishehsari et al. reported that initial GI symptoms found in 22.4% of SARS-CoV-2-positive patients were associated with worse outcomes [64][41]. Other researchers, such as Livanos et al., performed a high-dimensional analysis of GI tissues to assess the level of inflammation, measured by cytokines and chemokines gene expression (i.e., IFNG, CXCL8, CXCL2, and IL1B) and the abundance of pro-inflammatory cells. The authors also concluded that GI symptoms were associated with reduced disease severity and mortality [66][42].4.2. Liver Involvement during COVID-19
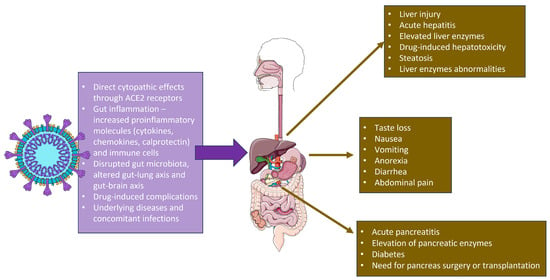
Even though COVID-19 is thought to be a systemic infection targeting mainly the endothelial cells and the respiratory tract, a more in-depth overview suggests the engagement of other organs and systems such as the liver, kidney, pancreas, and the heart, which makes SARS-CoV-2 a virus with multiple targets and complicated pathogenesis resulting in multiorgan failure in susceptible populations. It is believed that most pathogens causing activation of adaptive immune response include MHC1 molecules and T-cells, which is devastating for the respiratory tract and the hepatic cells [68][43]. A rare complication of cytokine storm could be liver dysfunction. However, liver damage may also occur due to the treatment, since many medications used to treat the infection are hepatotoxic. Ischemia, cytokine storm, and hypoxia were the primary variables contributing to liver injury during COVID-19. Elevated liver enzymes during hospitalization can be attributable to drugs, sepsis, and shock. As a result, the proportion of hospitalized patients with COVID-19 and pathological liver biomarkers ranges from 14% to 53%. In addition, aminotransferases (i.e., aspartate aminotransferase, AST, alanine aminotransferase, ALT) and bilirubin levels are frequently elevated. Elevated gamma-glutamyltransferase, alkaline phosphatase and reduced serum albumin levels are typically seen [69][44]. One possible explanation for these observations in the liver is that, although it is widely distributed in human tissues, ACE2 has the highest expression in the biliary epithelium. It has been proposed that SARS-CoV-2 can replicate in cholangiocytes. A rare finding in COVID-19 patients is cholangiopathy. However, a more common presentation regarding the liver is elevated levels of AST and ALT (as much as 40% in studies) and not the cholestatic GGT and AF. The aberrant immune response caused by SARS-CoV-2 infection in some patients, the direct injury caused by the hepatotropism of the virus, and the hepatotoxicity of some medications used during treatment are the main causes of liver damage. In patients with diagnosed liver diseases, COVID-19 may lead to acute worsening of symptoms. In cirrhotic patients, the ACE 2 expression increased, which explains the poorer outcome in that population and the less-than-desirable immune responses following vaccination [66][42]. Additionally, the Shanghai experience revealed that GI symptoms caused by Omicron variants are relatively uncommon. In the intestinal model, Kei et al. discovered that the Omicron variant strain had a lower proliferation efficiency. They measured viral RNA and titer in the culture supernatant and found that the Omicron versions had limited replication capability [77][45]. A large retrospective study of the Omicron outbreak in South Africa found that the hospitalization rate among adolescents and children was significantly higher than in previous outbreaks. Children under 20 years old accounted for 14.3% of all hospitalized patients, and about 25.4% of infected children under 5 years old required hospitalizations [78][46]. The hospitalization rate of children infected with Omicron was also reported to be high in the US, with 19% requiring ICU treatment [79][47]. Weak immunity, low vaccination rates, and increased respiratory illness rates in adolescents and children are all plausible causes. Poor outcomes have been found in patients with hepatocellular carcinoma and chronic liver disease. The cause behind liver injury in COVID-19 patients is multifactorial. It includes the pathogenic mechanism of the direct viral cytopathic effect and the upregulated immune response, and it results in vascular endothelitis, ischemia caused by the hypercoagulable state, concomitant hypoxia caused by the respiratory failure, and iatrogenic drug-induced liver injury [83][48]. The use of remdesivir and monoclonal antibodies, like tocilizumab and lopinavir/ritonavir, require careful assessment of previous liver disease and frequent monitoring of transferase enzymes; drug-induced liver injury (DILI) should be taken into consideration when treating COVID-19 patients [84][49]. A summary of the SARS-CoV-2 impact on the GI and hepatobiliary system is shown in Figure 1.
Figure 1. SARS-CoV-2 actions in the gastrointestinal tract and the relevant clinical symptoms. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
5. Clostridoides Difficile Infection as Gi Complication during COVID-19
As previously stated, antibiotic-associated diarrhea is a significant cause of poorer outcomes in COVID-19 patients. In 2022, Maslennikov et al. analyzed data from infected individuals. They concluded that coinfection with Clostridoides—one of the culprits behind antibiotic-associated diarrhea—negatively influenced the prognosis and course in SARS-CoV-2 infected individuals. The article stated that, in COVID-19 patients with diarrhea, Clostridoides should be considered and treated accordingly [89][50].
It has been hypothesized that a concomitant SARS-CoV-2 infection may lead to transitory immune dysregulation, making Clostridoides infection more common in COVID-19 patients. Another mechanism explaining the high prevalence of this infection in COVID-19 patients is mentioned above (i.e., a pathogenic mechanism involving the ACE2, altered gut microbiome, and antibiotic use) [90][51].
Moreover, the risk for GI disorders after COVID-19 could be related to gut microbiota alterations. This topic was determined by Xu et al. in 2023. Patients infected with the SARS-CoV-2 virus presented in the post-acute phase (30 days after infection) with signs and symptoms of GERD, acute gastritis, peptic ulcers, pancreatic (acute pancreatitis), biliary (cholangitis), and hepatic disorders, as well as functional abnormalities such as motility disorders, IBS, and functional dyspepsia [92][52]. The evidence suggests that GI symptoms are a part of the so-called “long COVID” in hospitalized and non-hospitalized patients.
In line with this, preventing infections and reinfections decreases the risk of GI disorders. It has been hypothesized that the pathogenic mechanisms behind the symptoms are due to the viral tropism to tissues in the GI tract, induced autoimmunity, chronic inflammation due to viral persistence with residual antigenic stimulation, and dysbiosis. In addition to the pathophysiology of COVID-19 sequelae, notable changes in the gut microbiome are present. Furthermore, the appendix is a site for continuous viral replication after infection.
6. Conclusions
Commonly observed symptoms during SARS-CoV-2 infection were associated with GI involvement, including diarrhea, nausea, vomiting, anorexia, and abdominal pain. However, disease signs differ from the first wave (Alpha) to the last wave (Omicron) regarding the symptomatic infection and symptoms from respiratory, GI, neurology, etc. Most studies demonstrated that the Omicron variant of SARS-CoV-2 was also associated with digestive symptoms, but this was less prominent than the previous virus variants. In contrast, Delta was associated with an increased risk for hospitalization due to COVID-19, mainly due to GI complications. As other GI infections, microbiota alterations, and pancreatic and liver involvement could deteriorate the overall prognosis of COVID-19 patients, it was established that preventing infections and reinfections decreased the risk of GI disorders and death.References
- Shi, Y.; Mei, Z.; Wang, H. Characteristics and implications of Omicron variant associated digestive system infections—Correspondence. Int. J. Surg. 2022, 104, 106750.
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of Omicron and Delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624.
- Wang, M.K.; Yue, H.Y.; Cai, J.; Zhai, Y.J.; Peng, J.H.; Hui, J.F.; Hou, D.Y.; Li, W.P.; Yang, J.S. COVID-19 and the digestive system: A comprehensive review. World J. Clin. Cases 2021, 9, 3796–3813.
- Velikova, T.V.; Kotsev, S.V.; Georgiev, D.S.; Batselova, H.M. Immunological aspects of COVID-19: What do we know? World J. Biol. Chem. 2020, 11, 14–29.
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based Design of Prefusion-stabilized SARS-CoV-2 Spikes. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science (New York, N.Y.) 2020, 369, 1501–1505.
- Velikova, T.; Snegarova, V.; Kukov, A.; Batselova, H.; Mihova, A.; Nakov, R. Gastrointestinal mucosal immunity and COVID-19. World J. Gastroenterol. 2021, 27, 5047–5059.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032.
- Berdowska, I.; Matusiewicz, M. Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis—With impact on gastrointestinal tract. World J. Gastroenterol. 2021, 27, 6590–6600.
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e3.
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919.
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481.
- Zhang, H.; Shao, B.; Dang, Q.; Chen, Z.; Zhou, Q.; Luo, H.; Yuan, W.; Sun, Z. Pathogenesis and Mechanism of Gastrointestinal Infection With COVID-19. Front. Immunol. 2021, 12, 674074.
- Venegas-Borsellino, C.; Sankararaman, S.; Roche, K.; Burns, J.; Landis, R.M. Impact of COVID-19 on the Intestinal Microbiome. Curr. Nutr. Rep. 2021, 10, 300–306.
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471.
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut-lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95.
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104.
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.S.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13.
- Borner, T.; Shaulson, E.D.; Ghidewon, M.Y.; Barnett, A.B.; Horn, C.C.; Doyle, R.P.; Grill, H.J.; Hayes, M.R.; De Jonghe, B.C. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020, 31, 351–362.e5.
- Andrews, P.L.R.; Cai, W.; Rudd, J.A.; Sanger, G.J. COVID-19, nausea, and vomiting. J. Gastroenterol. Hepatol. 2021, 36, 646–656.
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672.
- Megyeri, K.; Dernovics, Á.; Al-Luhaibi, Z.I.I.; Rosztóczy, A. COVID-19-associated diarrhea. World J. Gastroenterol. 2021, 27, 3208–3222.
- Balaphas, A.; Gkoufa, K.; Colucci, N.; Perdikis, K.C.; Gaudet-Blavignac, C.; Pataky, Z.; Carballo, S.; Ris, F.; Stirnemann, J.; Lovis, C.; et al. Abdominal pain patterns during COVID-19: An observational study. Sci. Rep. 2022, 12, 14677.
- Serban, D.; Tribus, L.C.; Vancea, G.; Stoian, A.P.; Dascalu, A.M.; Suceveanu, A.I.; Tanasescu, C.; Costea, A.C.; Tudosie, M.S.; Tudor, C.; et al. Acute Mesenteric Ischemia in COVID-19 Patients. J. Clin. Med. 2021, 11, 200.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496.
- Long, B.; Carius, B.M.; Chavez, S.; Liang, S.Y.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Clinical update on COVID-19 for the emergency clinician: Presentation and evaluation. Am. J. Emerg. Med. 2022, 54, 46–57.
- Wise, J. COVID-19: Symptomatic infection with omicron variant is milder and shorter than with Delta, study reports. BMJ 2022, 377, o922.
- Sinagra, E.; Shahini, E.; Crispino, F.; Macaione, I.; Guarnotta, V.; Marasà, M.; Testai, S.; Pallio, S.; Albano, D.; Facciorusso, A.; et al. COVID-19 and the Pancreas: A Narrative Review. Life 2022, 12, 1292.
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9.
- Lamers, M.M.; Beumer, J.; van der Vaart, J.; Knoops, K.; Puschhof, J.; Breugem, T.I.; Ravelli, R.B.G.; Paul van Schayck, J.; Mykytyn, A.Z.; Duimel, H.Q.; et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020, 369, 50–54.
- Lehmann, M.; Allers, K.; Heldt, C.; Meinhardt, J.; Schmidt, F.; Rodriguez-Sillke, Y.; Kunkel, D.; Schumann, M.; Böttcher, C.; Stahl-Hennig, C.; et al. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8+ T cells. Mucosal Immunol. 2021, 14, 1381–1392.
- Merola, E.; Armelao, F.; de Pretis, G. Prevalence of gastrointestinal symptoms in coronavirus disease 2019: A meta-analysis. Acta Gastroenterol. 2020, 83, 603–615.
- Parasa, S.; Desai, M.; Thoguluva Chandrasekar, V.; Patel, H.K.; Kennedy, K.F.; Roesch, T.; Spadaccini, M.; Colombo, M.; Gabbiadini, R.; Artifon, E.L.A.; et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011335.
- Schulze, H.; Bayer, W. Changes in Symptoms Experienced by SARS-CoV-2-Infected Individuals—From the First Wave to the Omicron Variant. Front. Virol. 2022, 2, 880707.
- Mocanu, V.; Bhagwani, D.; Sharma, A.; Borza, C.; Rosca, C.I.; Stelian, M.; Bhagwani, S.; Haidar, L.; Kshtriya, L.; Kundnani, N.R.; et al. COVID-19 and the Human Eye: Conjunctivitis, a Lone COVID-19 Finding—A Case-Control Study. Med. Princ. Pract. 2022, 31, 66–73.
- Thaweerat, W. Current evidence on pancreatic involvement in SARS-CoV-2 infection. Pancreatology 2020, 20, 1013–1014.
- Morris, A. Effects of pancreatic SARS-CoV-2 infection identified. Nat. Rev. Endocrinol. 2021, 17, 192.
- Scherer, P.E.; Kirwan, J.P.; Rosen, C.J. Post-acute sequelae of COVID-19: A metabolic perspective. eLife 2022, 11, e78200.
- Lee, M.H.; Li, H.J.; Wasuwanich, P.; Kim, S.E.; Kim, J.Y.; Jeong, G.H.; Park, S.; Yang, J.W.; Kim, M.S.; Yon, D.K.; et al. COVID-19 susceptibility and clinical outcomes in inflammatory bowel disease: An updated systematic review and meta-analysis. Rev. Med. Virol. 2023, 33, e2414.
- Hunt, R.H.; East, J.E.; Lanas, A.; Malfertheiner, P.; Satsangi, J.; Scarpignato, C.; Webb, G.J. COVID-19 and Gastrointestinal Disease: Implications for the Gastroenterologist. Dig. Dis. 2021, 39, 119–139.
- Bishehsari, F.; Adnan, D.; Deshmukh, A.; Khan, S.R.; Rempert, T.; Dhana, K.; Mahdavinia, M. Gastrointestinal Symptoms Predict the Outcomes From COVID-19 Infection. J. Clin. Gastroenterol. 2022, 56, e145–e148.
- Livanos, A.E.; Jha, D.; Cossarini, F.; Gonzalez-Reiche, A.S.; Tokuyama, M.; Aydillo, T.; Parigi, T.L.; Ladinsky, M.S.; Ramos, I.; Dunleavy, K.; et al. Intestinal Host Response to SARS-CoV-2 Infection and COVID-19 Outcomes in Patients With Gastrointestinal Symptoms. Gastroenterology 2021, 160, 2435–2450.e34.
- Bzeizi, K.; Abdulla, M.; Mohammed, N.; Alqamish, J.; Jamshidi, N.; Broering, D. Effect of COVID-19 on liver abnormalities: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10599.
- Taneva, G.; Dimitrov, D.; Velikova, T. Liver dysfunction as a cytokine storm manifestation and prognostic factor for severe COVID-19. World J. Hepatol. 2021, 13, 2005–2012.
- Miyakawa, K.; Machida, M.; Kawasaki, T.; Nishi, M.; Akutsu, H.; Ryo, A. Reduced replication efficacy of SARS-CoV-2 Omicron variant in “mini-gut” organoids. Gastroenterology. 2022, 163, 514–516.
- Jassat, W.; Abdool Karim, S.S.; Mudara, C.; Welch, R.; Ozougwu, L.; Groome, M.J.; Govender, N.; von Gottberg, A.; Wolter, N.; Wolmarans, M.; et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: A retrospective observational study. Lancet Glob. Health 2022, 10, e961–e969.
- Shi, D.S.; Whitaker, M.; Marks, K.J.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kawasaki, B.; Meek, J.; et al. Hospitalizations of children aged 5–11 Years with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 2020–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 574–581.
- Nasa, P.; Alexander, G. COVID-19 and the liver: What do we know so far? World J. Hepatol. 2021, 13, 522–532.
- Sodeifian, F.; Seyedalhosseini, Z.S.; Kian, N.; Eftekhari, M.; Najari, S.; Mirsaeidi, M.; Farsi, Y.; Nasiri, M.J. Drug-Induced Liver Injury in COVID-19 Patients: A Systematic Review. Front. Med. 2021, 8, 731436.
- Maslennikov, R.; Ivashkin, V.; Ufimtseva, A.; Poluektova, E.; Ulyanin, A. Clostridioides difficile coinfection in patients with COVID-19. Future Microbiol. 2022, 17, 653–663.
- Lakkasani, S.; Chan, K.; Shaaban, H.S. Clostridiodes difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020. Emerg. Infect. Dis. 2020, 26, 2299–2300.
- Xu, E.; Xie, Y.; Al-Aly, Z. Long-term gastrointestinal outcomes of COVID-19. Nat. Commun. 2023, 14, 983.
More
