Amyloidosis is a group of rare and underrecognised diseases characterised by the extracellular deposition of misfolded proteins that aggregate into insoluble amyloid fibrils
[1]. Amyloid fibrils can deposit in multiple organs and tissues, thus altering their structure and function. Amyloidosis can be systemic or localised, inherited or acquired, and different forms have been characterised according to the type of precursor protein
[2]. The three most frequent types of systemic amyloidosis that may involve the heart are: (i) monoclonal immunoglobulin light-chain (AL) amyloidosis; (ii) hereditary transthyretin (TTR) amyloidosis (ATTRv); and (iii) wild-type TTR amyloidosis (ATTRwt). AL amyloidosis is caused by a clonal plasma cell disorder, wherein the pathogenetic clone produces excess immunoglobulin light chains, resulting in amyloid formation and deposition
[3]. This form is characterised by an aggressive clinical course and poor survival, if not diagnosed early
[4]. ATTR amyloidosis stems from a misfolded TTR protein; ATTRwt amyloidosis is age-related and predominantly affects men > 70 years old, while the clinical manifestations of ATTRv are strongly related to the specific genetic variants
[5]. In all these forms, cardiac involvement and the severity of cardiac amyloidosis (CA) are the main determinants of prognosis
[6]. The cardinal symptom of patients with CA is heart failure with preserved ejection fraction (HFpEF)
[7][8][7,8]. However, patients may also frequently encounter conduction disorders and arrhythmias
[9][10][9,10]. Among the most clinically relevant complications of systemic amyloidosis are thrombotic and haemorrhagic events, particularly in CA patients
[11]. The mechanisms underlying these events are only partially known, most notably with regard to ATTR amyloidosis.
2. Interactions between Transthyretin and the Coagulation System
Transthyretin is a tetrameric protein produced primarily by the liver, with smaller quantities being produced by the choroid plexus and retinal pigmented epithelial cells; it functions as a transporter protein for thyroxine and retinol-binding proteins
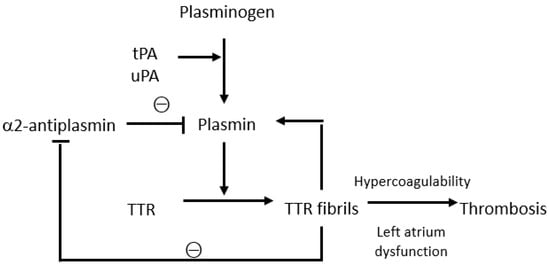
[12]. ATTR amyloid also appears to play a role in the activation and regulation of the coagulation and fibrinolytic system (
Figure 1). The pathogenesis of ATTR amyloidosis is a complex and not yet fully understood process, involving several mechanisms, and physiological fibrinolysis in vivo may be interconnected with amyloid formation. In fact, plasmin can cleave TTR, resulting in the formation of TTR fibrils containing truncated TTR molecules similar to the fibrils isolated from TTR amyloid formed in vivo. Notably, by measuring the levels of the plasmin–α2-antiplasmin complex, it has been demonstrated that a hyperfibrinolytic state is present mainly in patients with AA and AL amyloidosis, but not in those with TTR amyloidosis
[13]. TTR plays a more complex role as amorphous extracellular protein aggregates bind to plasmin, thus contributing to plasmin activation and protecting plasmin from α2-antiplasmin inhibition. There is a self-contained regulatory system involving plasmin activation by amyloid and amyloid degradation by plasmin
[14]. Circulating TTR can diffuse to the extracellular compartment and become trapped in or leak from the fibrin clot. Plasminogen is converted to plasmin by uPA in the extracellular compartment and by tPA in the clot. The binding of tPA and plasminogen to fibrin occurs in a lysine-dependent manner. Plasmin cleaves tetrameric TTR and releases a truncated residue 49–127 C-terminal fragment and full-length protomers that can generate fibrillar nuclei that aggregate into amyloid fibrils and accumulate in the extracellular space
[12]. The fibrinolytic system appears to be linked to the onset of spontaneous intracerebral haemorrhage (ICH) in patients with cerebral amyloid angiopathy (CAA) who present a pathological amyloid beta (Aβ) deposition in the brain vasculature (i.e., cortical and leptomeningeal arteries, arterioles, and capillaries). Mutated Aβ in hereditary cerebral amyloid angiopathy enhances tPA-mediated plasminogen activation, alters clot structure, delays fibrinolysis, and contributes to the haemorrhagic and ischaemic manifestations of CAA. Normally, plasminogen activation degrades both Aβ and fibrin. However, in the presence of excessive plasmin generation due to elevated Aβ and vascular wall damage due to Aβ deposition and alterations of the cerebral microvasculature, plasminogen activation may increase the risk of spontaneous ICH due to hyperfibrinolysis, complicating r-tPA thrombolysis in acute ischaemic stroke
[15]. In addition to the fibrinolytic system, TTR also plays a role in the coagulation system. Beta amyloid can bind to fibrinogen and cause fibrin clots to become structurally altered and resistant to fibrinolysis (protease-resistant amyloid–fibrin clot). X-ray crystallography revealed the binding of Abeta42 to the D-fragment of fibrinogen, which induces a structural change in the latter, thus blocking the cleavage of fibrin by plasmin
[16]. Therefore, TTR has been found in platelet-rich fibrin and the formation of functional TTR amyloid in coagulation processes may contribute to both blood clotting and wound healing. Furthermore, the binding of amyloid to fibrin delays the proteolysis of amyloid by plasmin, creating a balance between the activation of plasmin by amyloid and the degradation of amyloid by plasmin
[17]. TTR levels are also strongly associated with incident VTE, as shown in a case–control study that collected blood samples from 200 patients with ATTR before VTE events occurred. In a panel of 46 potential predictors of VTE, transthyretin was identified as the strongest biomarker with a nominal
p-value of 0.00015, and there is a dire correlation between plasma TTR levels and procoagulant activity in the blood
[18]. A few studies conducted in patients with Coronavirus disease 2019 (COVID-19) found that plasma TTR levels correlated directly with the hypercoagulable state responsible for thrombotic complications, with a simultaneous reduction in TTR degradation by plasmin
[19]. Abnormalities of the coagulation and fibrinolytic systems also appear to contribute to haemorrhagic events, as demonstrated in patients with familial amyloid polyneuropathy (FAP) who show significantly lower levels of prothrombin fragment 1 + 2 compared to the controls, though the role of transthyretin remains unclear
[20].
Figure 1.
Interactions between the fibrinolytic system and transthyretin. tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator; TTR, transthyretin.
3. Thrombotic Events in Transthyretin Amyloidosis
Thrombotic events are among the most important complications of both AL and ATTR amyloidosis, especially with cardiac involvement
[21][22][21,22]. However, studies that investigated the incidence, characteristics, and risk factors of thrombotic events in patients with ATTR amyloidosis are scarce and mostly include small single-centre cohorts. Intracardiac thrombi and cerebral ischemic events have been described among major thromboembolic events in ATTR amyloidosis, whereas peripheral and gastrointestinal thrombosis are less common
[21]. Patients with transthyretin amyloid cardiomyopathy (ATTR-CM) showed a high prevalence of atrial fibrillation (AF) (88%), which occurred more frequently in ATTR vs. AL. Amyloid infiltration of the atria and myocardium is thought to predispose patients to AF and high thromboembolic risk
[23][24][23,24]. To date, whether a hypercoagulable state may contribute to the thrombotic diathesis and its underlying mechanisms are unknown. There have been reports noting the high prevalence of intracardiac thrombosis not only in patients with AF but also those with a normal sinus rhythm. A study by Vilches et al.
[22] found an incidence rate of 0 per 100 patient-years among patients with a normal sinus rhythm undergoing oral anticoagulant treatment (OAT); 1.3 among those with a normal sinus rhythm without OAT; 1.7 among those with AF undergoing OAT; and 4.8 among those with AF without OAT. The amyloid infiltration of the left atrium may result in atrial mechanical dysfunction and endothelial damage with consequent blood flow stasis, in particular in the presence of atrial stiffness and the electromechanical dissociation of the left atrium
[23][24][23,24]. Moreover, diuretic therapy in the presence of concomitant heart failure is also thought to affect Virchow’s triad, causing abnormalities in factors involved in clotting and contributing to clot formation even in subjects with a normal sinus rhythm
[25]. Understanding the underlying mechanisms of thromboembolism, in particular whether the coagulation system is involved, is paramount to ascertain which patients would benefit from an early anticoagulation treatment. The latest European Society of Cardiology (ESC) consensus document on myocardial and pericardial diseases recommends anticoagulant therapy in all patients with AF regardless of the CHA2SD2-VASc score, but also in selected patients with a normal sinus rhythm
[26].
4. Bleeding Events in Transthyretin Amyloidosis
4.1. Spontaneous Bleeding Manifestations: Case Reports
Bleeding manifestations are relatively frequent in AL amyloidosis
[27][37]. However, several cases related to ATTR amyloidosis have been recently described, due to amyloid deposits in vessel walls causing the acceleration of microcalcification and vessel rupture. The most common dermatological findings are purpura and ecchymosis, caused by vascular fragility and wall damage possibly due to amyloid deposition and subsequent bleeding diathesis
[28][38]. Some case reports appear to confirm these findings, such as the case of a 75-year-old man hospitalised for heart failure who presented with periorbital ecchymosis (raccoon eye) and shoulder swelling (pad sign) who was eventually diagnosed with ATTRwt-CA by cardiac biopsy
[29][39]; and the case of a 69-year-old woman with periorbital purpura who, after echocardiography, was diagnosed with ATTRv-CA with the genetic variant Thr80Ala
[30][40]. There have also been reports in the literature of gastrointestinal bleeding, obstruction, or perforation as a possible result of amyloid deposition in the gastrointestinal mucosa; a 79-year-old man with intra-abdominal haemorrhage underwent emergency partial resection of the transverse colon and the postoperative pathological examination of tissue samples led to the diagnosis of ATTR amyloidosis
[31][41]. Urinary tract bleeding was reported in a patient with severe haematuria who was diagnosed with ATTRwt by cystoscopy and bladder biopsy. Amyloid can build up under the superficial mucosa or in the vasculature of the urinary tract, potentially causing massive bleeding
[32][42]. Cases of retinal haemorrhage also have been reported in patients with ATTR amyloidosis due to vascular endothelial cell damage
[33][34][43,44].
4.2. Bleeding While Undergoing DOACs vs. VKAs
Clotting abnormalities have been described mainly in AL amyloidosis, including prolonged prothrombin time, activated partial thromboplastin time, and FX deficiency; there are little data on ATTR amyloidosis
[21]. The bleeding tendency in patients with ATTR amyloidosis is generally less severe than that in patients with AL and AA, though anticoagulation can exacerbate it. Therefore, the potential benefits of anticoagulation must be weighed carefully against potential bleeding complications
[21][28][21,38]. A study by Mitrani RL et al.
[35][33] found no differences in the combined outcome of IS, TIA, major bleeding, or death in patients with ATTR-CA and AF treated with warfarin vs. DOACs. However, labile INR was observed in 87% of patients and the incidence rate of major bleeding events was 3.7 per 100 person-years in this study vs. 2.2–3.9 in the general population.
4.3. Type of Haemorrhagic Events According to Anticoagulant Therapy
4.3. Type of Haemorrhagic Events According to Anticoagulant Therapy
The most frequent haemorrhagic events are haemorrhagic strokes and major extracranial bleeding. These events may occur in patients undergoing anticoagulation with no differences between direct oral anticoagulants and warfarin, as highlighted by Bukhari et al.
[36], who reported haemorrhagic strokes in 4.4% and major extracranial bleeding in 7.3% of ATTRwt patients with AF. Major haemorrhagic events during anticoagulation were also reported by Vilches et al.
[22], who studied 273 patients with AF receiving VKAs and 216 receiving DOACs during a median follow-up of 14.2 months. Overall, 32 haemorrhagic events occurred: 18 with VKAs and 14 with DOACs; major gastrointestinal and intracranial bleedings occurred in 46.9% and 12.5% of cases, respectively.
4.4. Amyloid Angiopathy Associated with an Increased Fragility of Blood Vessels
Microcalcifications are a common occurrence in cardiac ATTR amyloidosis (CA-ATTR) that are irregularly distributed and most pronounced near the fibrous body. Therefore, they are not strictly associated with amyloid deposits but are often found in collagenous areas. These clouds of microcalcifications explain the cardiac uptake of the bone tracers observed in CA-ATTR scintigraphy with technetium-99
[37][46]. Notably, histopathology examinations, CT, and MRI have shown vascular microcalcifications in hereditary CAA, though the underlying mechanisms are not yet fully understood
[38][47]. These microcalcifications are most likely a secondary complication of CAA not directly caused by amyloid deposition and possibly induced by the extracellular osteopontin trapped in the fibrotic vessel wall. Vascular microcalcifications in amyloid-laden vessels may cause rupture, thus leading to both asymptomatic microbleeds and lobar ICH
[39][48]. This mechanism is comparable to that observed in calcific aortic valve disease (CAVD), wherein the presence of apolipoprotein-related amyloid deposits near calcified areas suggests a possible interplay between the development of aortic valve stenosis, amyloid deposition, and calcification. These amyloid deposits may indeed contribute to enhance the inflammatory cycle in the aortic valve, including the remodelling of the extracellular matrix and the proliferation of myofibroblast- and osteoblast-like cells. Chemical changes in proteins in the local proinflammatory environment surrounding amyloid deposits could also play a role in calcification
[40][49].