You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Frederick Kibenge and Version 2 by Peter Tang.
The influenza A virus (IAV) poses a significant global threat to public health and food security. Chile’s encounter with IAV began in 2002, with the highly pathogenic avian influenza (HPAI) H7N3 virus, derived from a unique South American low pathogenic avian influenza (LPAI) virus.
- influenza A virus
- avian influenza virus
- poultry farms
1. Introduction
Influenza viruses belong to the Orthomyxoviridae family [1], and, along with the Amnoonviridae family [2], they constitute the order Articulavirales [3]. Members of this family possess a segmented negative-sense single-stranded RNA genome. The number of genome segments varies, depending on the genus: Orthomyxoviridae has 6–8 segments, while Amnoonviridae has 10. Influenza viruses are classified into four genera: Alphainfluenzavirus, Betainfluenzavirus, Gammainfluenzavirus, and Deltainfluenzavirus. Each genus has a single ratified species: influenza A virus (IAV), influenza B virus (IBV), influenza C virus (ICV), and influenza D virus (IDV), respectively [4]. The Orthomyxoviridae family includes not only influenza viruses but also other orthomyxoviruses categorized into different genera, such as Thogotovirus, Isavirus, Mykissvirus, Sardinovirus, and Quarajavirus [1][4][5][6][1,4,5,6]. Most recently, the reclassification of the genus Quarajavirus as the family Quaranjaviridae has been proposed. Additionally, a novel and divergent family, tentatively named Cnidenomoviridae, has been discovered in Cnidaria (including corals) and is now included in the order Articulavirales, bringing the total number of families in this order to four [7].
2. AIV H7N3
The first IAV disease occurrence in commercial poultry in Chile was in 2002 and was caused by HPAI H7N3 (Figure 1), characterized as lineage A/chicken/Chile/184240-4322/2002(H7N3), which had mutated from an LPAI virus [8][21]. The epicenter of the outbreak was a broiler breeder farm in the densely populated poultry region of San Antonio, V Region, Chile. The farm consisted of 27 poultry sheds housing birds ranging from 1 to 79 weeks in age, besides a hatchery [9][22]. Between April and May 2002, the farm experienced a clinical disease characterized by a relatively low mortality rate, a slight decline in egg production, and cases of salpingo-peritonitis. In certain cases, mortality was so sudden that no clinical signs were observable. The necropsy revealed cyanotic combs and wattles, as well as petechial hemorrhages in various organs, such as the muscles, heart, pancreas, and legs. Subcutaneous edema was also present. Subsequent surveillance activities identified a second outbreak occurring one week later at a turkey breeding farm owned by the same company. This farm, situated 4 km from the initial outbreak, consisted of eight pens that housed turkeys ranging in age from 6 to 59 weeks, besides a hatchery. The infection was confined to 25% of the sheds. Clinical signs predominantly affected the upper respiratory tract and were followed by a sudden increase in mortality. Cloacal swabs were collected, and an HPAI virus was isolated from two samples from this farm, which were identical to those obtained from the index case [9][22]. The initial identification of LPAI H7N3 was followed by the detection of HPAI H7N3, which was accompanied by increased mortality rates. Detailed analysis of the viral genomes has yielded valuable insights, revealing minimal genetic differences between the low and highly pathogenic strains, except for a significant alteration in the cleavage site of the HA protein. The LPAI H7N3 virus exhibited a cleavage site similar to that of other LPAI H7 viruses. In contrast, the HPAI H7N3 isolates displayed a 30-nucleotide insertion at this specific site. This insertion possibly resulted from recombination events between the HA and nucleoprotein genes of the LPAI H7N3, leading to an increase in pathogenicity. A comparative analysis of the full sequences of the eight gene segments confirmed that the Chilean viruses had a distinct nature vis-a-vis other AIVs, forming a unique clade exclusive to South America. These findings highlight the critical importance of the continuous monitoring and surveillance of AIVs. These viruses can undergo mutations that significantly increase their pathogenicity [10][16]. In addition, identifying a distinct viral clade specific to South America underscores the necessity for an understanding of and control strategies tailored to effectively managing AIV outbreaks in the region [11][23].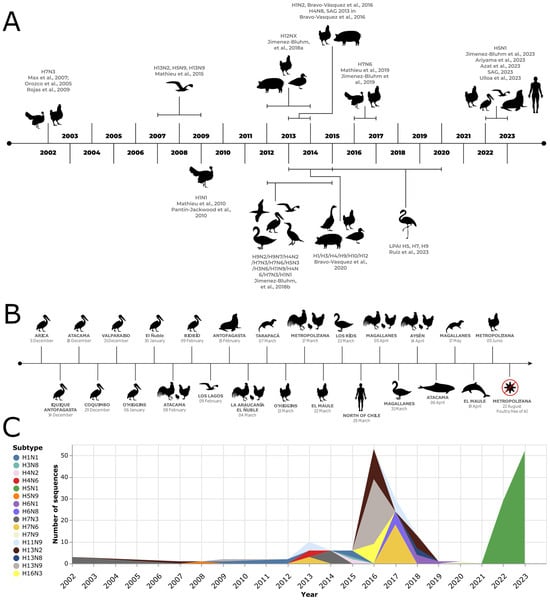
Figure 1. Influenza A virus outbreak in birds and non-human mammals in Chile. (A) Literature review derives a timeline of outbreaks [8][9][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][21,22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. (B) H5N1 cases were registered during late 2022 and 2023, based on data from the Chilean Agricultural and Livestock Service [24][36] and the National Fisheries and Aquaculture Service [28][40]. (C) Number of hemagglutinin sequences available from avian samples and classified by subtype per year in Chile. Only the three most abundant subtypes per year are shown. Sequences retrieved from the NCBI nucleotide database [29][41].
