Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Patrycja Jastrząb.
The aberrant sialylation of membrane glycocalyx plays a pivotal role in the regulation of malignant cell behavior and correlates with a worse prognosis and shorter overall survival for patients. The biological and physical properties of sialome determine the negative charge and high hydrophilicity of cell membranes and thereby regulate cell–cell and cell–extracellular matrix interactions. There is increasing evidence that sialic acids influence cellular susceptibility in therapeutic management.
- sialic acid
- sialome
- cancer
1. Introduction
According to the concept by Cohen and Varki, cell membrane glycomolecules form a dense network characterized by a high grade of diversity and dynamic variability [1]. It makes these structures like a forest with complex “tree crowns” formed by glycans covalently conjugated to protein or a lipid core. The complex structure and function of the glycome depend on monosaccharide units and the stereospecific formation of glycosidic bonds that determine their combination and the spatial organization of glycans. Indeed, the glycoconjugate landscape is dynamically formed by glycome–metabolic enzyme and glycome–metabolic pathway modifying factors that result in specific glycosylation patterns and their critical role in molecular and cellular biology. As a result of metabolic conversion, the activated monosaccharide units of dietary or molecular carbohydrate recycling origin are assembled into complex sugar chains in the Golgi apparatus and attached to the protein or lipid core via glycosyltransferases [2,3][2][3] (Figure 1).
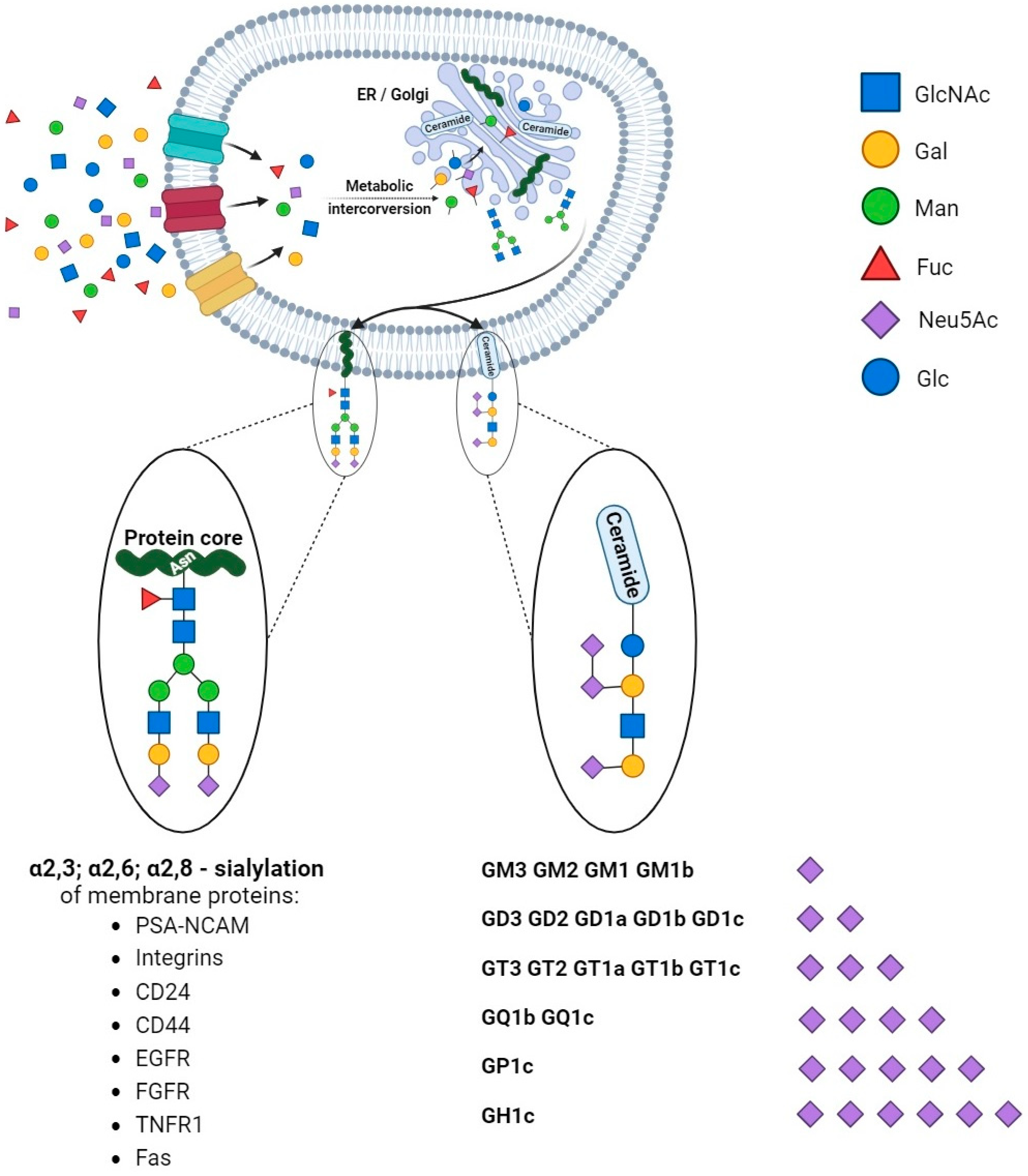
Figure 1. Synthesis and diversity of glycoproteins and glycolipids. The monosaccharide units are translocated into the cells and undergo modifications via phosphorylation and conjugation with nucleotides. The sequential assembly of glycoproteins and glycolipids is orchestrated through essential collaboration between the endoplasmic reticulum (ER) and the Golgi apparatus. Initial modifications occur in the ER, followed by subsequent refinements within the Golgi apparatus. In physiological and pathological conditions, proteins undergo sialylation, including those pivotal in cancer processes. Additionally, monosaccharides form intricate structures with lipids, exhibiting varying quantities of sialic acids (in the figure, ranked from the least to the most sialylated).
Structural studies on cellular glycomes have revealed the glycan epitopes of defined sequences featured in the wide expression of neuraminic acid derivatives routinely called sialic acids. The attachment of sialic acids to the non-reducing end of sugar chains is the last step of glycosylation and results in enhanced microheterogeneity that influences the physical and biochemical properties of glycoconjugates [4]. The specific location, ubiquity, and varied forms of these molecules make them one of the most versatile molecules participating in many physiological processes. They are of particular importance in the field of cell–cell and cell–extracellular matrix interactions that underlie biological recognition, cellular communication, adhesion, and migration. The high-specific sialoglycans have been shown to modulate the function of cell membrane receptors via alterations in dimerization, activation, and autophosphorylation that result in changes in cellular signaling and thereby regulate cell proliferation and survival [5]. The proper sialylation pattern is balanced via the activities of sialyltransferases and sialidases that play crucial roles in the maintenance of cellular homeostasis. Therefore, the ability to affect cellular processes makes sialylation a key player in human health in the fields of nephrology, hepatology, neurobiology, endocrinology, cardiovascular physiology, reproductive processes, and immune responses [6]. Advances in glycobiology and glycoengineering have revealed that the unbalanced synthesis and metabolism of sialoglycans is a pathological risk factor and predictive factor for cell and tissue dysfunction. Finally, aberrant sialylation has been described as a potential therapeutic target and modulator of conventional therapy efficiency. Molecular and clinical studies have confirmed the participation of sialic acids in human pathology; however, the role of sialoglycans in cancer deserves particular attention. Clinical and molecular investigations have confirmed alterations in the sialylation patterns of tumors of various histological types and origins. As shown, the presence of hypersialylated glycans is crucial for malignant cells’ behavior, particularly their activity in a tumor niche, cross-talk with surrounding normal tissue, and infiltrating immune cells [7]. This variation modulates immunosurveillance, making the cells more resistant to the immune response. Indeed, sialic-acid-related immune evasion is a hallmark of many malignancies closely associated with a poor prognosis [4]. Moreover, there is increasing evidence of the engagement of sialic acids in the drug resistance phenomenon in cancer management [8]. Although the modulatory effects of sialic acids on the drug action have been described, the exact mechanisms by which an altered sialylation pattern contributes to drug resistance in cancer are not yet fully understood. Both a poor therapeutic response and multidrug resistance are serious clinical problems; therefore, understanding the modulatory role of sialome in drug sensitivity seems to be important for the development and/or improvement of therapeutic efficiency.
2. Sialic Acids and the Regulation of Cellular Functions in Cancer
Sialome plays a complex and multifaceted role in the structure of cell membranes and the extracellular matrix; therefore, numerous alterations in this field are known to be critical for cancer progression. Similar to normal cells, the decoration of the cellular glycocalyx via sialic acids can impact cellular recognition, signaling, adhesion, molecular trafficking, and migration. However, cancer cells do not express the molecules on their membrane properly, and altered glycosylation and changes in glycoprotein expression are common features of tumorigenesis [9]. Among molecular variations, increased sialylation is a hallmark of several cancers, including breast, colorectal, lung, and brain tumors [7,10,11,12,13,14][7][10][11][12][13][14]. Many functions in cells and tissues are regulated via ionic flows, electric fields, and voltage gradients, so any aberrations that cause changes in charge will affect them [15]. Sialic acids are known to create a negative charge on the cell membrane that influences the hydrophilicity of the cell surface and regulates cellular adhesiveness. It is of particular importance in cell migration and determines the metastatic potential of malignant cells [7]. As shown, the overexpression of sialic-acid-terminated glycans leads to alterations in immune status and antibody functions that facilitate tumor formation and correlate with aggressiveness [7,16][7][16]. Among sialylated adhesion modulators, the polymers of alpha 2,8-linked sialic acid (polysialic acid, PSA) affect the adhesive properties of neuronal cell adhesion molecules (NCAMs) and perform a pivotal function in the dynamic neuronal generation in the embryonic and neonatal hippocampus [17,18,19,20][17][18][19][20]. The repulsive effects caused by PSA allow cells to migrate, as has been confirmed in various types of cancer [17,21,22][17][21][22]. Sialic-acid-dependent cell migration is potentiated via PSA in a hypoxic environment; however, it is still unknown how oxygen deficiency affects glycosylation and sialylation in cancer [23,24][23][24]. In addition to polysialylated NCAMs (PSA-NCAMs), the family of selectins is involved in adhesion processes during cancer progression. The interplay between selectins and their ligands, sialyl-Lewis-X (sLeX), promotes interactions between cancer cells and endothelial cells, leukocytes, or platelets and thereby plays an important role in metastasis via facilitating the extravasation of tumor cells from the bloodstream [25]. The meta-analysis by Liang et al. has shown that sialyl-Lewis-X overexpression is associated with cancer metastasis, disease recurrence, and overall survival in cancer patients [26]. The exact reasons for altered sialylation in cancer are still unknown. However, three probable mechanisms that may influence changes in sialome are indicated [14]. The first mechanism underlying hypersialylation may be associated with altered metabolic flux via cancer cells with the ability to modulate their metastatic potential. Metabolic reprogramming is one of the hallmarks of cancer, and the reprogrammed metabolism supports its development. Moreover, there are several reports dealing with the influence of metabolic flux on sialylation. The investigation by Almaraz et al. has indicated that the malignancy status becomes altered due to massive metabolic flux through the sialic acid pathway. As shown, the uptake of sialic acid precursors in tumor cells from the extracellular space results in a significant increase in glycoproteins sialylation [27].3. Enzymatic Dysregulation of Sialylated Glycocalyx in Cancer
Changes in the sialylation controlling machinery were found in several types of cancers and described as markers of sialome-related tumorigenesis. First, the aberrant sialylation processes in malignancies correspond to the altered expression and activity of endogenous sialidases in diverse cellular compartments. Among the four sialidases (NEU1, NEU2, NEU3, and NEU4), the enhanced expression of NEU3 was observed in malignant tissues, whereas the rest of these enzymes tended to be reduced, which led to the accumulation of sialoconjugates [14]. According to Miyagi et al., the altered sialidases of different subcellular localizations play pivotal roles in controlling transmembrane signaling, lysosomal catabolism, and the modulation of functional molecules involved in many biological processes [28]. Besides the sialidases, the family of glycosyltransferases is part of the complex mechanism underlying changes in glycosylation patterns that are closely related to human pathologies, including the pathology of cancer [29]. The transfer of sialic acid to the appropriate acceptors is catalyzed via the group of 20 sialyltransferases that are observed to be raised in many malignancies [30]. As a result, the overexpression of sialoglycans and their incorrect branching promote tumor migration and invasion through the influence of cancer cell angiogenesis, adhesion, and epithelial–mesenchymal transition (EMT) [31]. These effects are strongly correlated with the advanced stage of malignancy, high metastatic potential, and patients’ reduced overall survival [32,33,34][32][33][34] (Figure 2A,B).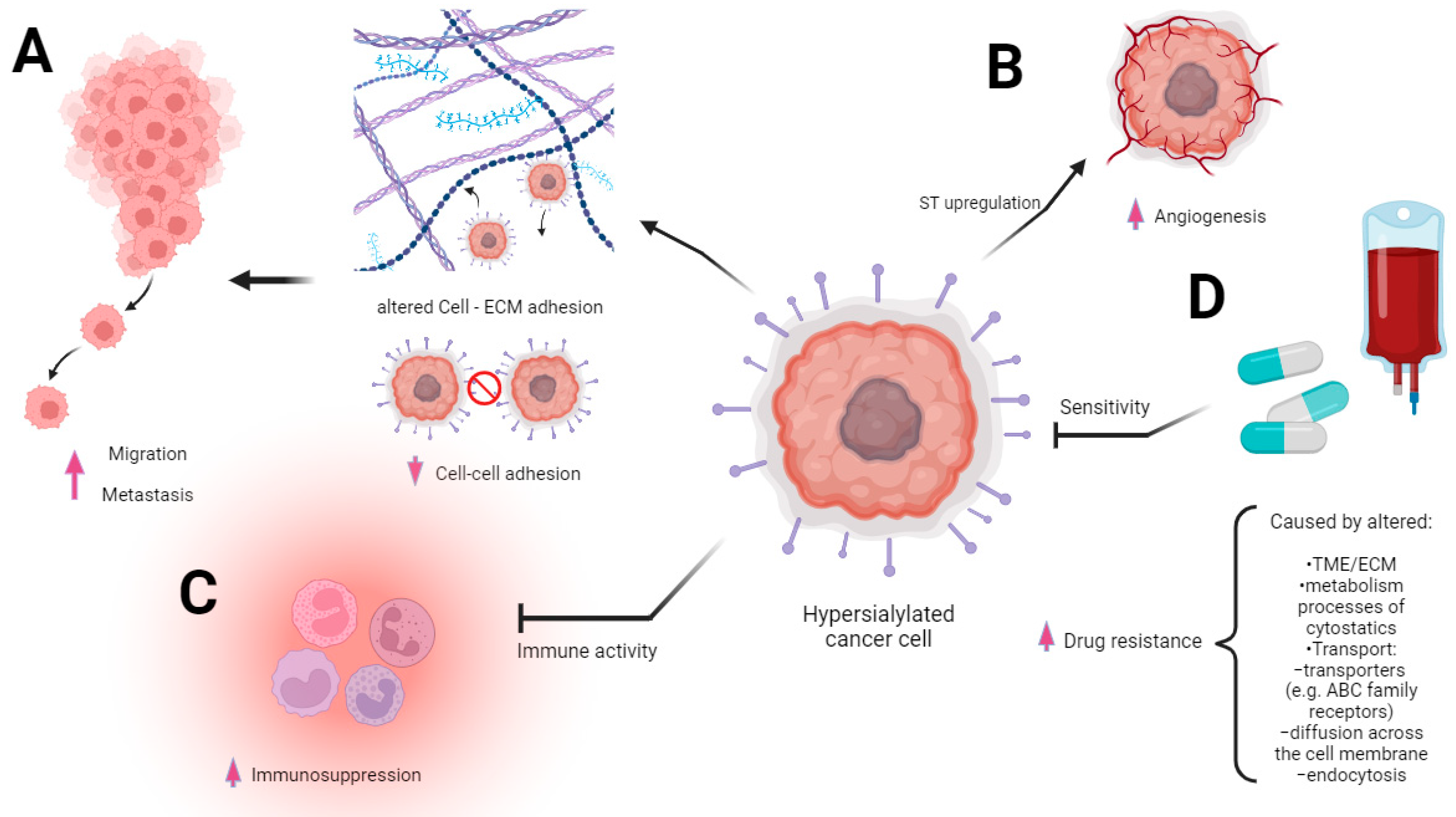
Figure 2. Multidirectional involvement of sialic acid overexpression in cancer progression. The figure underscores the multifaceted role of sialic acid overexpression in driving various aspects of cancer progression. The modified extracellular matrix (ECM) within the tumor microenvironment is altered via the heightened presence of sialic acid. This change in ECM composition fosters significant alterations in the migratory behavior of cancer cells (A). The heightened immunosuppression is mediated via sialic acid overexpression. Sialic acids and sialic acid binding receptors participate in evading immune surveillance and thereby facilitating cancer progression (B). The heightened level of sialic acids, contributing to the augmentation of angiogenesis processes, is intricately associated with alterations in ST expression. The sialic acids’ presence is intricately linked to an upsurge in angiogenic signals and fosters a conducive environment for tumor vascularization, culminating in enhanced vessel growth (C). Drug resistance may ensue from the coexistence of diverse mechanisms intricately linked to carcinogenesis with a high likelihood of multiple biochemically mediated pathways contributing to the emergence of resistance. Sialic acids’ overexpression is involved in altering drug influx and efflux mechanisms, a driving factor behind this acquired resistance (D).
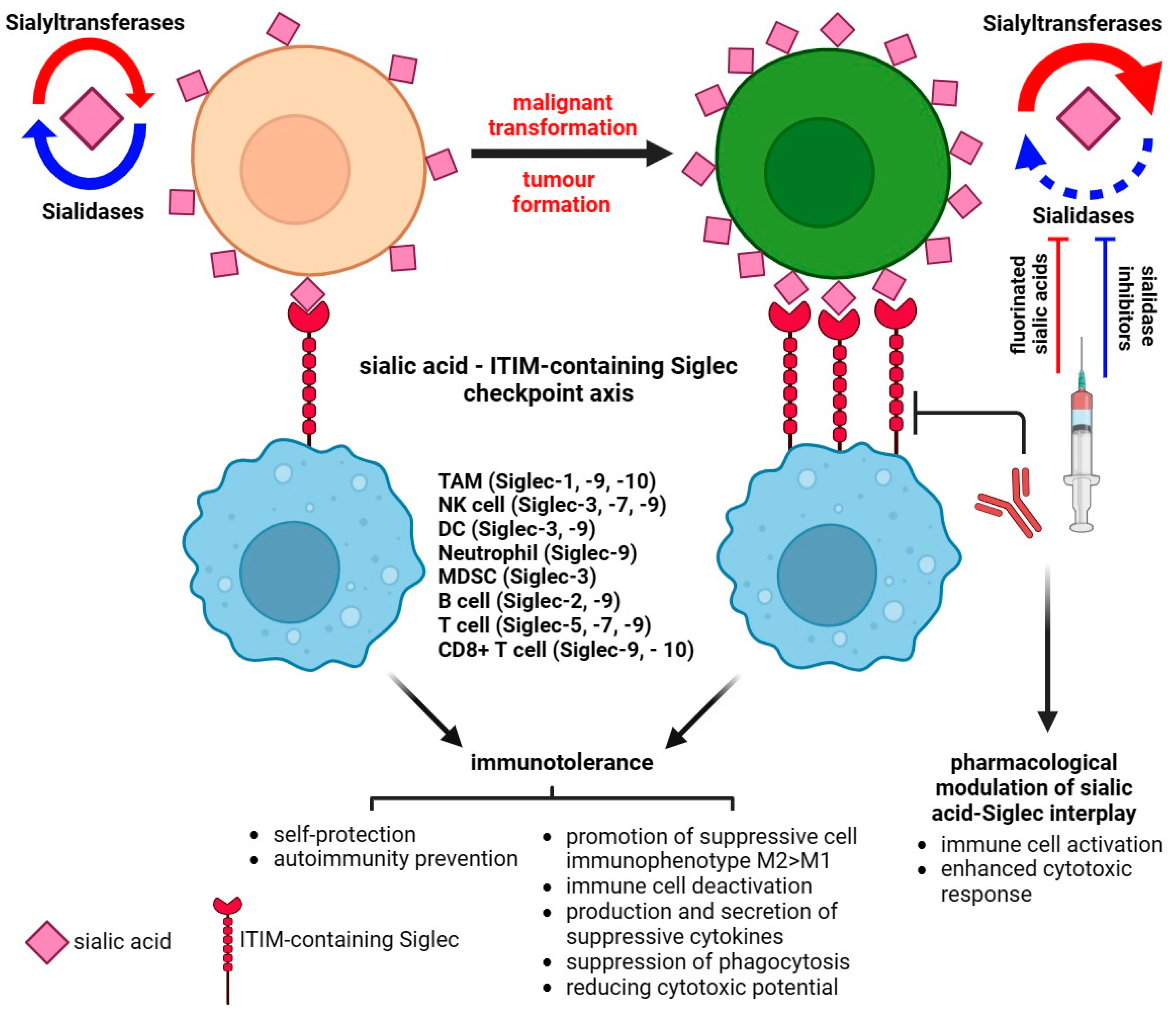
Figure 3. Sialic acid–Siglec checkpoint in immune control in health and cancer. The interplay between membrane sialoglycans and inhibitory Siglecs results in immunotolerance and the promotion of cancer progression. The pharmacological targeting of Siglec or sialome-modulatory machinery disturbs the immunosuppressive status of the tumor microenvironment and impairs the invasive potential of cancer.
References
- Cohen, M.; Varki, A. The sialome—Far more than the sum of its parts. Omics 2010, 14, 455–464.
- Bertozzi, C.R.; Kiessling, L.L. Chemical glycobiology. Science 2001, 291, 2357–2364.
- Dutta, H.; Jain, N. Post-translational modifications and their implications in cancer. Front. Oncol. 2023, 13, 1240115.
- Ghosh, S. Sialic acid and biology of life: An introduction. In Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease; Academic Press: Cambridge, MA, USA, 2020; pp. 1–61.
- Ankenbauer, K.E.; Rao, T.C.; Mattheyses, A.L.; Bellis, S.L. Sialylation of EGFR by ST6GAL1 induces receptor activation and modulates trafficking dynamics. J Biol Chem. 2023, 299, 105217.
- Fliniaux, I.; Marchand, G.; Molinaro, C.; Decloquement, M.; Martoriati, A.; Marin, M.; Bodart, J.F.; Harduin-Lepers, A.; Cailliau, K. Diversity of sialic acids and sialoglycoproteins in gametes and at fertilization. Front. Cell Dev. Biol. 2022, 10, 982931.
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90.
- Munkley, J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers 2022, 14, 4248.
- Pinho, S.; Reis, C. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555.
- Pally, D.; Pramanik, D.; Hussain, S.; Verma, S.; Srinivas, A.; Kumar, R.V.; Everest-Dass, A.; Bhat, R. Heterogeneity in 2,6-Linked Sialic Acids Potentiates Invasion of Breast Cancer Epithelia. ACS Cent. Sci. 2021, 7, 110–125.
- Seales, E.C.; Jurado, G.A.; Brunson, B.A.; Wakefield, J.K.; Frost, A.R.; Bellis, S.L. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005, 65, 4645–4652.
- Kondo, K.; Harada, Y.; Nakano, M.; Suzuki, T.; Fukushige, T.; Hanzawa, K.; Yagi, H.; Takagi, K.; Mizuno, K.; Miyamoto, Y.; et al. Identification of distinct N-glycosylation patterns on extracellular vesicles from small-cell and non-small-cell lung cancer cells. J. Biol. Chem. 2022, 298, 101950.
- Yamamoto, H.; Oviedo, A.; Sweeley, C.; Saito, T.; Moskal, J.R. Alpha2,6-sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer Res. 2001, 61, 6822–6829.
- Büll, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204.
- Ghirardello, M.; Shyam, R.; Galan, M.C. Reengineering of cancer cell surface charges can modulate cell migration. Chem. Commun. 2022, 58, 5522–5525.
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an Important Regulator of Antibody Function. Front. Immunol. 2022, 13, 818736.
- Mindler, K.; Ostertag, E.; Stehle, T. The polyfunctional polysialic acid: A structural view. Carbohydr. Res. 2021, 507, 108376.
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology 2005, 15, 887–894.
- Fujimoto, I.; Bruses, J.L.; Rutishauser, U. Regulation of cell adhesion by polysialic acid. Effects on cadherin, immunoglobulin cell adhesion molecule, and integrin function and independence from neural cell adhesion molecule binding or signaling activity. J. Biol. Chem. 2001, 276, 31745–31751.
- Gascon, E.; Vutskits, L.; Kiss, J.Z. Polysialic acid–neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res. Rev. 2007, 56, 101–118.
- Yang, P.; Yin, X.; Rutishauser, U. Intercellular space is affected by the polysialic acid content of NCAM. J. Cell Biol. 1992, 116, 1487–1496.
- Hoffman, S.; Edelman, G.M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA 1983, 80, 5762–5766.
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70.
- Elkashef, S.M.; Allison, S.J.; Sadiq, M.; Basheer, H.A.; Ribeiro Morais, G.; Loadman, P.M.; Pors, K.; Falconer, R.A. Polysialic acid sustains cancer cell survival and migratory capacity in a hypoxic environment. Sci. Rep. 2016, 6, 33026.
- Borsig, L. Selectins in cancer immunity. Glycobiology 2018, 28, 648–655.
- Liang, J.X.; Liang, Y.; Gao, W. Clinicopathological and prognostic significance of sialyl Lewis X overexpression in patients with cancer: A meta-analysis. Onco Targets Ther. 2016, 9, 3113–3125.
- Almaraz, R.T.; Tian, Y.; Bhattarcharya, R.; Tan, E.; Chen, S.H.; Dallas, M.R.; Chen, L.; Zhang, Z.; Zhang, H.; Konstantopoulos, K.; et al. Metabolic flux increases glycoprotein sialylation: Implications for cell adhesion and cancer metastasis. Mol. Cell. Proteom. 2012, 11, 017558.
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896.
- Miyagi, T.; Takahashi, K.; Hata, K.; Shiozaki, K.; Yamaguchi, K. Sialidase significance for cancer progression. Glycoconj. J. 2012, 29, 567–577.
- Gc, S.; Bellis, S.L.; Hjelmeland, A.B. ST6Gal1: Oncogenic signaling pathways and targets. Front. Mol. Biosci. 2022, 9, 962908.
- Xu, C.; Wang, S.; Wu, Y.; Sun, X.; Yang, D.; Wang, S. Recent advances in understanding the roles of sialyltransferases in tumor angiogenesis and metastasis. Glycoconj. J. 2021, 38, 119–127.
- Dorsett, K.A.; Marciel, M.P.; Hwang, J.; Ankenbauer, K.E.; Bhalerao, N.; Bellis, S.L. Regulation of ST6GAL1 sialyltransferase expression in cancer cells. Glycobiology 2021, 31, 530–539.
- Orozco-Moreno, M.; Visser, E.A.; Hodgson, K.; Hipgrave Ederveen, A.L.; Bastian, K.; Goode, E.A.; Öztürk, Ö.; Pijnenborg, J.F.A.; Eerden, N.; Moons, S.J.; et al. Targeting aberrant sialylation and fucosylation in prostate cancer cells using potent metabolic inhibitors. Glycobiology 2023, cwad085.
- Wichert, B.; Milde-Langosch, K.; Galatenko, V.; Schmalfeldt, B.; Oliveira-Ferrer, L. Prognostic role of the sialyltransferase ST6GAL1 in ovarian cancer. Glycobiology 2018, 28, 898–903.
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis. Onc. 2019, 3, 7.
- Greville, G.; McCann, A.; Rudd, P.M.; Saldova, R. Epigenetic regulation of glycosylation and the impact on chemo-resistance in breast and ovarian cancer. Epigenetics 2016, 11, 845–857.
- Peixoto, A.; Relvas-Santos, M.; Azevedo, R.; Santos, L.L.; Ferreira, J.A. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front. Oncol. 2019, 9, 380.
- Li, C.; Teixeira, A.F.; Zhu, H.J.; Ten Dijke, P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer 2021, 20, 154.
- Barallobre-Barreiro, J.; Baig, F.; Fava, M.; Yin, X.; Mayr, M. Glycoproteomics of the Extracellular Matrix: A Method for Intact Glycopeptide Analysis Using Mass Spectrometry. J. Vis. Exp. 2017, 122, 55674.
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028.
- Bassagañas, S.; Pérez-Garay, M.; Peracaula, R. Cell surface sialic acid modulates extracellular matrix adhesion and migration in pancreatic adenocarcinoma cells. Pancreas 2014, 43, 109–117.
- Coulson-Thomas, Y.M.; Gesteira, T.F.; Norton, A.L.; Kao, W.W.; Nader, H.B.; Coulson-Thomas, V.J. The role of proteoglycans in the reactive stroma on tumor growth and progression. Histol. Histopathol. 2015, 30, 33–41.
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406.
- Dennis, J.; Waller, C.; Timpl, R.; Schirrmacher, V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature 1982, 300, 274–276.
- Suzuki, O.; Abe, M.; Hashimoto, Y. Sialylation and glycosylation modulate cell adhesion and invasion to extracellular matrix in human malignant lymphoma: Dependency on integrin and the Rho GTPase family. Int. J. Oncol. 2015, 47, 2091–2099.
- Dasgupta, D.; Pally, D.; Saini, D.K.; Bhat, R.; Ghosh, A. Nanomotors Sense Local Physicochemical Heterogeneities in Tumor Microenvironments. Angew. Chem. Int. Ed. 2020, 59, 23690–23696.
- Yu, S.; Fan, J.; Liu, L.; Zhang, L.; Wang, S.; Zhang, J. Caveolin-1 up-regulates integrin α2,6-sialylation to promote integrin α5β1-dependent hepatocarcinoma cell adhesion. FEBS Lett. 2013, 587, 782–787.
- Su, C.Y.; Li, J.Q.; Zhang, L.L.; Wang, H.; Wang, F.H.; Tao, Y.W.; Wang, Y.Q.; Guo, Q.R.; Li, J.J.; Liu, Y.; et al. The Biological Functions and Clinical Applications of Integrins in Cancers. Front. Pharmacol. 2020, 11, 579068.
- Yuan, Y.; Wu, L.; Shen, S.; Wu, S.; Burdick, M.M. Effect of alpha 2,6 sialylation on integrin-mediated adhesion of breast cancer cells to fibronectin and collagen IV. Life Sci. 2016, 149, 138–145.
- Seales, E.C.; Jurado, G.A.; Singhal, A.; Bellis, S.L. Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene 2003, 22, 7137–7145.
- Shaikh, F.M.; Seales, E.C.; Clem, W.C.; Hennessy, K.M.; Zhuo, Y.; Bellis, S.L. Tumor cell migration and invasion are regulated by expression of variant integrin glycoforms. Exp. Cell Res. 2008, 314, 2941–2950.
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131.
- Sasano, T.; Gonzalez-Delgado, R.; Muñoz, N.M.; Carlos-Alcade, W.; Cho, M.S.; Sheth, R.A.; Sood, A.K.; Afshar-Kharghan, V. Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer. J. Thromb. Haemost. 2022, 20, 104–114.
- Kaneko, M.K.; Oki, H.; Hozumi, Y.; Liu, X.; Ogasawara, S.; Takagi, M.; Goto, K.; Kato, Y. Monoclonal Antibody LpMab-9 Recognizes O-glycosylated N-Terminus of Human Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2015, 34, 310–317.
- Kaneko, M.K.; Kato, Y.; Kameyama, A.; Ito, H.; Kuno, A.; Hirabayashi, J.; Kubota, T.; Amano, K.; Chiba, Y.; Hasegawa, Y.; et al. Functional glycosylation of human podoplanin: Glycan structure of platelet aggregation-inducing factor. FEBS Lett. 2007, 581, 331–336.
- van Houtum, E.J.H.; Büll, C.; Cornelissen, L.A.M.; Adema, G.J. Siglec Signaling in the Tumor Microenvironment. Front. Immunol. 2021, 12, 790317.
- Gianchecchi, E.; Arena, A.; Fierabracci, A. Sialic Acid-Siglec Axis in Human Immune Regulation, Involvement in Autoimmunity and Cancer and Potential Therapeutic Treatments. Int. J. Mol. Sci. 2021, 22, 5774.
- Crocker, P.; Paulson, J.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266.
- Jiang, K.Y.; Qi, L.L.; Kang, F.B.; Wang, L. The intriguing roles of Siglec family members in the tumor microenvironment. Biomark. Res. 2022, 10, 22.
- Hudak, J.; Canham, S.; Bertozzi, C. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75.
More
