Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Theo Gulen.
Mast cells (MCs), as multifunctional immune cells, orchestrate the typical allergic conditions wherein the activation of these cells by allergens, including pollen, food, medication, and the venom of stinging insects, leads to the degranulation and elaboration of the inflammatory mediators responsible for regulating the acute dramatic inflammatory response. Anaphylaxis, mast cell activation syndrome (MCAS), and mastocytosis are interrelated yet distinct conditions within the spectrum of mast cell activation disorders.
- anaphylaxis
- MCAS
- mastocytosis
1. Mast Cells and Mast Cell Activation
Mast cells (MCs) are granulated cells that have a widespread distribution in all vascularized tissues [1,2,3,4,5][1][2][3][4][5]. The multifunctional capacity, heterogeneity, and plasticity of MCs enable them directly or indirectly to regulate innate and adaptive immune responses by communicating with other cells in the immune system [4,5,6][4][5][6]. The local microenvironment directly affects their maturation, phenotype, function, and ability to respond to internal or external stimuli by releasing biologically active mediators [4,5][4][5].
MCs express numerous surface receptors and are involved in the initiation and perpetuation of allergic inflammation. MCs can be activated by various mechanisms, most often through the cross-linking of immunoglobulin E (IgE) molecules bound to their surface by high-affinity FcεRI receptors [3,8,9][3][7][8]. Non-IgE-mediated mechanisms leading to MC activation include activation pathways through toll-like receptors, stem cell factor receptors (KIT = CD117), complement receptors C3a and C5a, and surface G protein-coupled receptors, including MRGPRX2 [9,20][8][9]. When activated, in a sequential order, MCs release various biologically active mediators [1,8,9][1][7][8] (Figure 1).
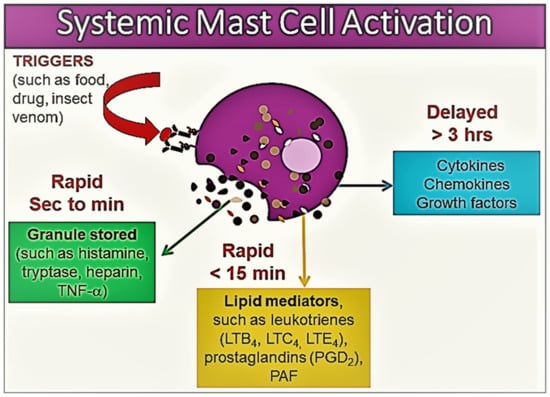
Figure 1. An illustration of the release of various mast cell mediators in the context of systemic mast cell activation. The mast cell inflammatory mediator profile shows heterogeneity according to the tissue microenvironment, the severity of mast cell activation, and the release of mast cell products. Please see the related text for further discussion.
Within minutes of activation, MC degranulation leads to the release of the preformed mediators stored in the MC granules, including histamine, serotonin, heparin, chondroitin sulphate, tryptase, chymase, carboxypeptidase, and TNF-α [1,8,9,10][1][7][8][10]. This first stage is followed by the de novo synthesis of membrane lipid-derived mediators, particularly prostaglandin (PG) D2, cysteinyl leukotrienes (LTC4, D4, and E4), and platelet activating factor (PAF) [8,9,10][7][8][10]. At the next stage, MC activation results in the synthesis of a variety of pro- and anti-inflammatory cytokines, including TNF-α, GM-CSF, IL-1, IL-3, IL-4, IL-5, IL-6, IL-13, IL-1RA, chemokines (such as IL-8, CCL-2, CC-3, CCL-5, and CXCL-8), growth factors (such as transforming growth factor-beta 1 (TGF-β1), stem cell factor (SCF), fibroblast growth factor (FGF), nerve growth factor (NGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF)), and interferons [1,8,9,10][1][7][8][10] (Figure 1).
The roles of mediators differ in the clinical manifestations of MC disorders, and related symptoms may show great heterogeneity according to the tissue in which MC activation occurs, as well as the trigger that causes the MC activation [10]. MC-derived mediators, such as histamine, leukotrienes, prostanoids, and PAF, regulate vascular instability and the barrier dysfunction of endothelial cells, and contribute to oedema formation, hypovolemia, and shock [10,21,22][10][11][12]. Histamine, specifically, can contribute to many classic manifestations associated with acute allergic reactions such as pruritus, flushing, nausea, gastric hypersecretion, nasal congestion, and wheezing. Histamine has been shown to bind to four receptors: H1 (responsible for airway and mucosal inflammation, attention, sleep–wake cycle regulation, and food or water intake), H2 (involved in the relaxation of the airway and blood vessel smooth muscle and in gastric acid secretion), H3 (involved in neuroregulation and mediator release modifying cognition, sleep–wake cycle regulation, and inflammation), and H4 (involved in the modulatory effects on inflammatory responses) [10]. Furthermore, histamine, kinins, leukotrienes, and PAF may be responsible for the opening of endothelial gap junctions, resulting in angioedema, pulmonary edema, and gastrointestinal symptoms (such as crampy abdominal pain and diarrhea related to mucosal swelling). Histamine, PGD2, leukotrienes, PAF, and prostanoids are responsible for wheezing and mucus hypersecretion [10,21,22][10][11][12]. Cytokines, chemokines, leukotrienes, and histamine may lead to neurological symptoms including headaches, fatigue, a sense of impending doom, and confusion [10,21,22][10][11][12]. Studies suggest that the activation of the contact system and the secretion of the plasminogen activator and heparin may lead to coagulation abnormalities and bleeding. Likewise, blood basophils may also participate in allergies through FcεRI-dependent reactions by releasing a similar profile of mediators [1]. However, not all hypersensitivity reactions may involve basophils, even if the reaction is systemic [1]. Moreover, some of the relevant mediators and repair molecules are produced and released exclusively by MCs but not by basophils [1].
2. Disorders Associated with Mast Cell Activation and Nomenclature
Evidence in the recent literature suggests that the spectrum of disorders related to mast cell activation is broad and includes IgE-dependent allergic inflammation and other immunologic and inflammatory reactions. Activated MCs not only, however, participate in the pathogenesis of hypersensitivity disorders but are also involved in an emerging group of conditions, so-called mast cell activation disorder (MCAD), such as mastocytosis [12,23][13][14]. Pathologic MC activation is a key finding in both hypersensitivity and MCAD, albeit caused by entirely different mechanisms. Therefore, patients with both disorders present with overlapping symptomatology due to inappropriate MC mediator release (Figure 2). Notably, both exogenously triggered allergies and endogenously triggered MCAD may cause anaphylaxis, which can be described as a “unique” condition representing a common clinical feature of these two distinct conditions [12][13].
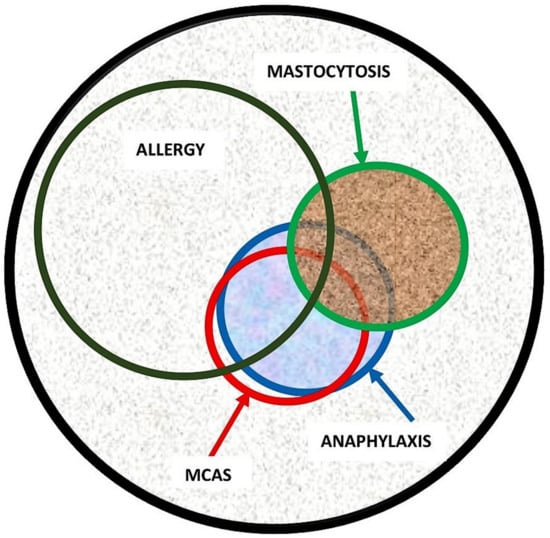
Figure 2. Illustration of disorders related to pathologic mast cell activation and association between allergy, anaphylaxis, mast cell activation syndrome (MCAS), and mastocytosis. While there is a significant overlap between the conditions, the size of the circle does not accurately represent the true percentage of overlapping. For example, the estimated overlap between anaphylaxis and mastocytosis is approximately 35%. However, there is currently a lack of systematic studies providing a precise estimation of the overlap between anaphylaxis and MCAS.
The signs and symptoms of disorders associated with MC activation and mediator release may range from tissue-specific events such as localized itching or nasal congestion to more systemic symptoms that result from widespread MC activation. Examples of the tissue-specific consequences of MC activation include urticaria, allergic rhinitis, or asthma, and the symptoms, in most instances, are limited to the area of interaction with the trigger, although generalized-tissue-specific symptoms also are possible, such as in the case of chronic idiopathic urticaria. Systemic MC activation reactions, such as anaphylaxis and MCAS, present with symptoms including two or more organ systems (skin: urticaria, angioedema, and flushing; gastrointestinal: nausea, vomiting, diarrhea, and abdominal cramping; cardiovascular: hypotensive syncope or near-syncope and tachycardia; upper and lower respiratory: conjunctival injection, nasal pruritus, nasal stuffiness, rhinorrhea, dyspnoea, and wheezing).
It is worth mentioning the nomenclature once again here. Experts often do not speak the same language, as they either use different terms for the same concepts, or the same term for different concepts, probably without being aware of it. For instance, the terms “mast cell activation disorder” (MCAD) and “mast cell activation syndrome” (MCAS) are often used interchangeably in the literature. Although they can refer to similar conditions involving abnormal MC activation, there are certain differences in their usage and scope. Although MCAD is a broader term encompassing a range of conditions characterized by pathologic MC activation, MCAS is a specific type of MCAD that is characterized by severe, recurrent episodes of systemic MC activation [12,23][13][14]. MCAS typically involves the release of excessive mast cell mediators throughout the body, resulting in a wide range of symptoms affecting multiple organ systems as mentioned in detail below in the MCAS section of this paper.
2.1. Mastocytosis
Mastocytosis refers to a complex heterogeneous multisystem disorder characterized by a pathologic activation and accumulation of clonally aberrant MCs in one or more organs, including the skin, bone marrow, liver, spleen, lymph nodes, and gastrointestinal tract [11,18][15][16]. The existing evidence suggests that it is a rare condition, and in recent studies, its prevalence is estimated to be 1 in 10,000 persons [24,25,26,27][17][18][19][20]. In general, mastocytosis can be divided into cutaneous mastocytosis (CM), where only the skin is affected, and systemic mastocytosis (SM), involving at least one additional organ other than the skin [11,18][15][16]. CM is the main form of the disease in children and patients are generally observed to have the onset of the disease within the first year of life. The most common form of skin involvement is maculopapular cutaneous lesions, also known as urticaria pigmentosa (UP) [11,18][15][16]. Pathognomonic Darier’s sign is a key feature and criterion of cutaneous lesions in mastocytosis; it is defined by swelling and redness after stroking or rubbing of the lesional skin in an affected individual. The sensitivity of Darier’s sign is over 90%. However, a skin biopsy should be obtained in doubtful cases and increased numbers of MCs in the upper dermis with a predilection to perivascular areas can confirm diagnosis.
Mastocytosis in children is most often limited to the skin and the prognosis is good, as skin lesions are resolved in most patients by adolescence. By contrast, patients with adult-onset mastocytosis have a persistent disease and may or may not present with skin lesions. In the majority of adult patients with maculopapular lesions (most frequently monomorphic, i.e., UP), MC infiltrates are also found in the bone marrow, corresponding to the final diagnosis of SM [11,18][15][16]. SM is diagnosed according to the World Health Organization (WHO) criteria, which consist of one major and four minor criteria, and the diagnosis requires demonstration of the major criterion (multifocal aggregates of MCs) along with at least one minor criterion, or three minor criteria in extracutaneous, most often bone marrow, biopsy materials (Table 1) [11,18][15][16]. Otherwise, adult patients with the typical skin lesions of mastocytosis are denoted as the mastocytosis in the skin (MIS) [18][16]. This is a provisional entity that is reserved for adult cases with cutaneous involvement (typically monomorphic maculopapular cutaneous lesions, i.e., UP), but in whom SM has not been ruled out yet. Although rare, there are also adult patients with skin lesions who do not show systemic involvement. In adult patients with true CM, the criteria for SM are not fulfilled, even if clonal MCs can be detected in the extracutaneous organs [18][16].
The most prevalent form of SM in adults is indolent SM (ISM) [26,27][19][20]. These patients have a normal life expectancy compared to the age-matched general population [26,27][19][20]; however, they may experience a variety of unpleasant symptoms. For instance, increased susceptibility to the development of malign melanoma has been reported in ISM patients [28][21]. In addition, advanced variants of SM, including aggressive SM (ASM), SM with associated hematologic neoplasm (SM-AHN), and MC leukemia (MCL), can occur in rare cases [18][16]. These patients generally have a large burden of clonally aberrant MCs in their bone marrow and carry a poor prognosis [18][16].
SM is a complex disease with diverse clinical manifestations ranging from asymptomatic disease to a highly aggressive course with multisystem involvement [11][15]. Most patients with ISM have symptoms caused by the inappropriate release of MC mediators, such as histamine, proteases (e.g., tryptase, chymase, and carboxypeptidase), and lipid-derived mediators (e.g., cysteinyl leukotrienes and prostaglandin D2) [11][15]. Symptoms may be acute or chronic and result from the local or remote effects of these mediators, which may act on multiple organ systems to induce so-called MC mediator release symptoms. Patients may present with diverse clinical findings, including flushing, pruritus, palpitations, dizziness, hypotension, syncope, breathing difficulties, abdominal pain, nausea, vomiting, diarrhea, headache, sweating, lethargy, fatigue, impaired concentration, irritability, anxiety, depression, arthralgia, myalgia, and osteoporosis [11][15]. However, not all patients experience all these manifestations; therefore, this heterogeneity is still unexplained. The mediator levels do not usually show a clear association with the clinical phenotypes, although the baseline levels of mediators including tryptase, histamine, and prostaglandin D2 are generally elevated [29][22]. Nevertheless, a history of flushing is a cardinal symptom [30][23]. In addition, some subjects may experience isolated symptoms, whereas others develop a constellation of signs and symptoms indistinguishable from that of anaphylaxis, which can be life-threatening [19,30][23][24]. Typically, patients suddenly feel very warm and then experience palpitations, dizziness, and a decrease in blood pressure due to systemic vasodilatation, which often leads to syncope [30][23]. Acute attacks may be brief or prolonged, but the duration is usually 15 to 30 min [30][23]. Patients often experience severe fatigue lasting around 24 h following spells [30][23]. Although specific triggers causing MC mediator release symptoms vary greatly among patients, exogenous and endogenous triggers may include physical exertion, cold, heat, insect venoms, the consumption of alcohol, infections, nonsteroidal anti-inflammatory drugs (NSAIDs), and emotional stress [11][15].
Advanced SM patients may also have symptoms of MC mediator release; interestingly, however, the occurrence of anaphylaxis due to the excessive release of MC mediators is less common compared with that in patients with ISM. These patients mainly experience symptoms due to MC infiltration and uncontrolled accumulation, including cytopenia, hepatosplenomegaly, lymph adenopathy, liver dysfunction, ascites, osteolytic bone lesions, and pathologic weight loss [11][15].
Table 1. Diagnostic criteria of systemic mastocytosis (SM) and monoclonal mast cell activation syndrome (MMAS) (adapted from references: [11,18,31,32][15][16][25][26]). Altogether, there is one major criterion and four minor criteria.
| SM | Diagnosis is confirmed if patient expresses one major criterion and one minor criterion or expresses three minor criteria in extracutaneous organ biopsy specimens |
| Major criterion Minor criteria |
|
| MMAS | Diagnosis requires presence of one or two minor criteria of SM |
|
2.2. Monoclonal Mast Cell Activation Syndrome
Monoclonal mast cell activation syndrome (MMAS) is a recently introduced variant of clonal MC disorders [31,32][25][26] and most such patients experience severe anaphylaxis episodes presenting with profound cardiovascular manifestations such as hypotension and syncope in the absence of urticaria. Although patients with MMAS have detectable clonal MCs expressing the D816V mutation and/or CD25+ aberrant markers, they do not fulfill the WHO criteria for SM diagnosis [11,18][15][16]. In addition, MMAS patients lack the typical skin changes in mastocytosis (Table 1). Furthermore, these patients have a normal to low burden of MCs and their serum baseline tryptase (sBT) levels generally range from 10 to 20 ng/mL but may also be within normal ranges.
The diagnosis of MMAS requires a high degree of clinical suspicion and confirmation by means of bone marrow biopsy. A diagnosis should be considered in patients presenting with symptoms of hypotensive anaphylaxis. Peripheral blood KIT D816V mutational analysis with an allele-specific PCR method can be used as a screening tool in these patients; however, the absence of this mutation in the peripheral blood does not rule out MMAS. Hence, a bone marrow biopsy should be considered in highly suspected cases.
This condition continues to exhibit limited understanding in terms of its natural course and prognosis and the development of comorbidities such as osteoporosis. Although some of these patients may be found to have SM in future biopsies, this is most probably because of misdiagnosis in the initial biopsies. According to personal observations, most MMAS patients do not progress into SM. Moreover, the spontaneous resolution of MMAS has not been described to date.
Another relevant point to highlight here is that MMAS and MCAS are related but distinct terms. These somewhat incongruous entities may complicate the nomenclature further. However, in certain patients with MMAS, MCAS may coexist when the diagnostic criteria for both conditions are met simultaneously (please see the MCAS criteria below in Section 3.3).
2.3. Anaphylaxis
Anaphylaxis is a common medical emergency and a life-threatening acute systemic hypersensitivity reaction that may lead to death by airway obstruction or cardiovascular collapse if not promptly treated. Anaphylaxis is one of the most well-known examples of systemic MC activation disorders and is caused by the abundant release of diverse MC mediators, leading to a constellation of varied symptoms from different organ systems [12,13][13][27]. The term anaphylaxis encompasses both IgE-mediated reactions and non-IgE-mediated mechanisms. Although this difference may impact allergen counselling, it has no importance in the acute management of the patient.
The data regarding the incidence and prevalence of anaphylaxis are somewhat inconsistent due to the fact that a consensus regarding its exact definition does not currently exist [33][28]. Studies from the USA suggest an incidence of up to 40–50 people per 100,000 person-years [34[29][30],35], whereas studies from Europe suggest a lower incidence of 1.5–7.9 per 100,000 person-years [36,37][31][32]. Moreover, the lifetime prevalence of anaphylaxis has been estimated to be approximately 0.3% [38][33]. There are studies showing an increase in admissions with anaphylaxis over the last two decades [38][33]. Although rare, deaths may also occur at a rate of 1 per three million population per year [39][34]. Foods, drugs, and the venom of stinging insects appear to be the most common elicitors of anaphylaxis, although the prevalence of triggers varies in children and adults. Food-induced anaphylaxis is the most common cause in children corresponding to 80–92% of anaphylaxis [40][35], whereas Hymenoptera venom and drug-induced anaphylaxis are the dominating elicitors among adults [41][36]. It should be also noted that although most anaphylactic reactions occur rapidly, delayed reactions, with onset up to 10 h after ingestion, may occur for some food allergens, e.g., with an alpha-Gal-induced red meat allergy [33][28].
Anaphylaxis concurrently affects multiple organ systems, and its diagnosis may be challenging, as the fine line differentiating an allergic reaction from anaphylaxis is not always easily discernible. Furthermore, the lack of globally recognized diagnostic criteria of anaphylaxis has not only long caused the failure of recognition and delayed treatment in patients but also hampered research facilities. Recently, however, multinational, multidisciplinary symposia were convened to achieve an international consensus on the clinical criteria for the diagnosis of anaphylaxis [42][37]. Accordingly, the current diagnostic criteria require the concurrent occurrence of symptoms from at least two organ systems that are related to the cutaneous, gastrointestinal, respiratory, and cardiovascular systems. The required organ system involvement varies depending on whether there is a “likely” or “known” trigger for the actual patient. Exceptionally, in the context of a confirmed allergy (e.g., insect venom or drug) for the given patient, an anaphylaxis diagnosis can be made only due to cardiovascular system involvement (hypotension and/or syncope) after re-exposure to the allergen. Additionally, even when there is no likely cause of the reactions, as in unprovoked anaphylaxis, when the onset of illness is acute, a diagnosis of anaphylaxis can be still made when either reduced blood pressure (or associated symptoms, such as syncope) and/or respiratory compromise or laryngeal oedema are present, accompanied by the involvement of skin–mucosal tissue symptoms [42][37]. The diagnostic criteria have been widely adopted and validated both retrospectively [43][38] and prospectively [44][39]. They were found to be 95% sensitive and 71% specific in a prospective validation study among emergency department patients [44][39]. Hence, it is critical for emergency department providers to consider anaphylaxis in the differential diagnosis for patients whose symptoms overlap with those of anaphylaxis, including upper airway obstruction, acute asthma, angioedema, flushing, hypotension, syncope, as delayed treatment with epinephrine may cause unexpected adverse outcomes, including fatality.
At present, anaphylaxis remains a clinical entity and its understanding for an allergist is still limited with respect to factors determining its severity and underlying intracellular mechanisms. However, existing clinical observations support the notion that anaphylaxis comprises a heterogeneous group of conditions regarding the nature and route of exposure to triggers, organ involvement, severity, and time course [12][13]. For instance, food-induced anaphylaxis is a leading cause in children, although venom- or drug-induced anaphylaxis account for the majority of adult cases. The distinctions are not only limited to triggers; even the clinical manifestations differ. Mortality is rare in children and occurs mostly in adult patients due to cardiovascular failure. Fatal anaphylaxis has also been associated with a lack of cutaneous symptoms during the anaphylactic episode.
To overcome these obstacles and recognize severe vs. milder reactions, there have been several attempts to develop severity grading systems for acute allergic reactions, including anaphylaxis [45,46,47,48][40][41][42][43]. However, they have not been widely implemented due to the lack of a uniformly accepted, validated grading system. Thus, there is currently an unmet need for an appropriately developed and validated severity scoring system for acute allergic reactions to harmonize clinical care and facilitate research.
2.4. Mast Cell Activation Syndrome
Mast cell activation syndromes (MCASs) represent a heterogeneous group of disorders that may have clonal or nonclonal etiologies. MCAS may be diagnosed when the symptoms of MC activation are systemic (involving more than one organ system), severe, and recurrent and the MCAS criteria are fulfilled [15,16,17][44][45][46]. There are three sets of criteria required for an MCAS diagnosis as illustrated in Figure 3: (1) the presence of typical, severe, episodic MC activation symptoms in ≥2 organ systems, including cutaneous, cardiovascular, gastrointestinal, and upper/lower respiratory symptoms; (2) the detection of a substantial transient increase in a validated marker of MC activation during the symptomatic event; (3) the control of symptoms with MC mediator-targeting drugs [15,16,17][44][45][46].
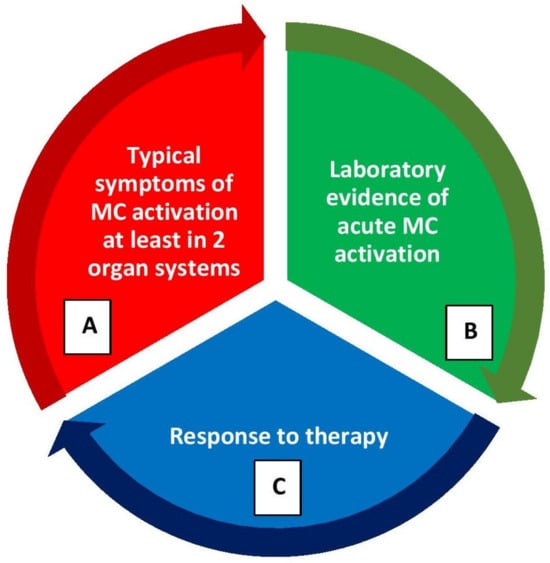
Figure 3. Diagnostic criteria for mast cell activation syndrome (MCAS) (please refer the text for further explanation). (A) Clinical criterion, (B) laboratory criterion, (C) response criterion. All three criterion should be fulfilled to confirm a diagnosis of MCAS. After the diagnosis, patients should be further evaluated for the classification of MCAS.
The best validated surrogate marker of MC activation is tryptase [14][47]. The tryptase levels obtained within 4 h of a suspected MC activation episode should be compared with the sBT (i.e., a recent tryptase sample drawn prior to the event or within 48 h after the event) levels. A formula of 1.2 × sBT + 2 ng/mL is used to calculate the minimal increase required to diagnose MC activation [17,49][46][48]. For instance, if the sBT level is 15 ng/mL, a level within 4 h of an MCAS episode of 20 ng/mL would be considered significant according to the above-mentioned formula [16,17,49][45][46][48]. Optimally, at least two such elevations should be considered diagnostic following acute, recurrent episodes; however, in practice, this is not always achievable and even one such measurement may be satisfactory.
When tryptase levels are not available, other validated mediators of MCs, such as urinary metabolites of histamine, prostaglandin D2, and leukotriene C4, can be measured in a 24 h or spot collection of the urine specimens collected after the patient empties their bladder during an acute event to confirm MC activation [17][46]. However, the sensitivity and specificity of these markers, as well as the minimal increases and cut-off levels diagnostic for MC activation, have not yet been established [17][46]. Recently, it has been suggested that levels greater than 30% above the upper limits of normal can be considered pathologic [50][49]. Nevertheless, assays are difficult to perform and only available in a few limited laboratories. Moreover, unlike tryptase, the other tests have not been standardized or compared in well-conducted studies.
Finally, MCAS diagnosis also requires a favorable response to agents that act as MC stabilizers or inhibitors of MC mediators. These include histamine receptor antagonists (H1- and H2-antihistamines), leukotriene blockers, MC stabilizers, and aspirin or nonsteroidal anti-inflammatory agents (NSAIDs) [12,51,52][13][50][51]. Selected cases may require immune suppression or cytoreductive therapy as well, especially for patients suffering from aggressive variants of mastocytosis [12,51,52][13][50][51].
Once a diagnosis of MCAS has been confirmed, further classification is necessary. MCAS has been classified into three variants [16,17][45][46]. Some patients with MCAS may have concurrently clonal MCs in bone marrow as in mastocytosis (systemic or cutaneous) or MMAS [16,17][45][46]. Patients with clonal mast cell disorders generally have varying degrees of expansion of the MC compartment derived from a progenitor with a genetic defect that presumably reduces the cell’s threshold for activation. These patients may have elevated sBT levels, carry KIT D816V mutations in lesional MCs, or have other markers of MC clonality, such as aberrant CD25 expression. Such MCAS patients are considered to have primary (i.e., clonal) MCAS and its diagnosis can only be made after an extracutaneous biopsy, most often after a bone marrow biopsy [16,17][45][46]. Thus, patients with clonal MCAS are required to fulfill the diagnostic criteria of both MCAS and clonal MC disease.
Nevertheless, the majority of patients with symptoms due to episodic, recurrent MC activation have non-clonal disorders. Thus, secondary MCAS results in symptoms of MC activation through IgE-mediated (such as food-, drug-, or Hymenoptera-venom-induced anaphylaxis) and non-IgE-mediated processes. These patients are cared for by allergists/immunologists. Moreover, in occasional cases, a patient with severe, recurrent MC activation may have an unremarkable work-up for allergic causes and have no evidence of clonal MC disease (usually ruled out after a bone marrow examination). These patients are considered for diagnoses of idiopathic anaphylaxis (IA) or idiopathic (non-clonal) MCAS, depending on the criteria patients fulfill. Furthermore, in some other cases, a primary MC activation (clonal) disorder may coexist with secondary MCAS (e.g., IgE-mediated hypersensitivity/MC reactions to food, medication, or stinging insect venoms). These patients are categorized as having combined or mixed MCAS.
In general, the signs and symptoms of recurrent IgE-mediated anaphylaxis may be the initial presentation of secondary or combined MCAS, whereas IA, e.g., unprovoked anaphylaxis can consist of the initial symptoms of clonal or idiopathic MCAS. However, it is worth mentioning here that not all anaphylaxis episodes fulfill the diagnostic criteria of MCAS, nor do all MCAS episodes reach the severity of anaphylaxis. For instance, some patients with systemic MC activation may have a lesser severity of symptoms that do not meet the definition of anaphylaxis. A typical such scenario is mastocytosis patients with unprovoked flushing episodes associated with abdominal pain. It is more appropriate that such patients are considered for a diagnosis of clonal MCAS rather than IA, as opposed to a patient who experiences hypotensive syncope or respiratory compromise combined with flushing episodes [53][52].
Although the diagnostic criteria and classification for MCAS have been established by an international (European Union-/US-based) consensus group during the last decade [15[44][45][46],16,17], there is still an ongoing debate about the use of the term MCAS in various groups of patients, and controversies remain. For instance, many patients with suspected MCAS and signs of MC activation do not fulfill the MCAS criteria [17][46]. In these patients, MC activation may be suspected as the major clinical problem, but it is a significant challenge to prove with certainty that the clinical features and symptoms are indeed derived from MC-dependent reactions and mediator release. Some of these patients may suffer from MC activation disorders (MCADs) or non-specified MC activation reactions, or attributed symptoms may not all be related to MC activation [23][14]. In these patients, local MC activation, less severe MC activation, or MC activation potentially involving only a limited set of mediators or only one organ system may be implicated, whereas other patients do not have an MCAD. Thus, it should be emphasized here that MCAS cannot be diagnosed in patients presenting with less severe and/or chronic symptoms of MC activation, as only clinically relevant symptoms of severe, systemic, and episodic MC activation can be classified as having MCAS [23][14].
References
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768.
- Crivellato, E.; Ribatti, D.; Mallardi, F.; Beltrami, C.A. The mast cell: A multifunctional effector cell. Adv. Clin. Path. 2003, 7, 13–26.
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786.
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044.
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738.
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529.
- Iwaki, S.; Tkaczyk, C.; Metcalfe, D.D.; Gilfillan, A.M. Roles of adaptor molecules in mast cell activation. Chem. Immunol. Allergy 2005, 87, 43–58.
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851.
- Kelso, J.M. MRGPRX2 signaling and skin test results. J. Allergy Clin. Immunol. Pract. 2020, 8, 426.
- Castells, M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 465–485.
- Butterfield, J.H. Nontryptase Urinary and Hematologic Biomarkers of Mast Cell Expansion and Mast Cell Activation: Status 2022. J. Allergy Clin. Immunol. Pract. 2022, 10, 1974–1984.
- Parente, R.; Giudice, V.; Cardamone, C.; Serio, B.; Selleri, C.; Triggiani, M. Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation. Int. J. Mol. Sci. 2023, 24, 7071.
- Gülen, T.; Akin, C. Anaphylaxis and Mast Cell Disorders. Immunol. Allergy Clin. N. Am. 2022, 42, 45–63.
- Valent, P.; Hartmann, K.; Bonadonna, P.; Gülen, T.; Brockow, K.; Alvarez-Twose, I.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Global Classification of Mast Cell Activation Disorders: An ICD-10-CM–Adjusted Proposal of the ECNM-AIM Consortium. J. Allergy Clin. Immunol. Pract. 2022, 10, 1941–1950.
- Gulen, T.; Hagglund, H.; Dahlen, B.; Nilsson, G. Mastocytosis: The puzzling clinical spectrum and challenging diagnostic aspects of an enigmatic disease. J. Intern. Med. 2016, 279, 211–228.
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated diagnostic criteria and classification of mast cell disorders: A consensus proposal. Hemasphere 2021, 5, e646.
- van Doormaal, J.J.; Arends, S.; Brunekreeft, K.L.; van der Wal, V.B.; Sietsma, J.; van Voorst Vader, P.C.; Oude Elberink, J.N.; Kluin-Nelemans, J.C.; van der Veer, E.; de Monchy, J.G. Prevalence of indolent systemic mastocytosis in a Dutch region. J. Allergy Clin. Immunol. 2013, 131, 1429–1431.e1.
- Cohen, S.S.; Skovbo, S.; Vestergaard, H.; Kristensen, T.; Møller, M.; Bindslev-Jensen, C.; Fryzek, J.P.; Broesby-Olsen, S. Epidemiology of systemic mastocytosis in Denmark. Br. J. Haematol. 2014, 166, 521–528.
- Zanotti, R.; Bonifacio, M.; Isolan, C.; Tanasi, I.; Crosera, L.; Olivieri, F.; Orsolini, G.; Schena, D.; Bonadonna, P. A Multidisciplinary Diagnostic Approach Reveals a Higher Prevalence of Indolent Systemic Mastocytosis: 15-Years’ Experience of the GISM Network. Cancers 2021, 13, 6380.
- Ungerstedt, J.; Ljung, C.; Klimkowska, M.; Gülen, T. Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience. Cancers 2022, 14, 3942.
- Hägglund, H.; Sander, B.; Gülen, T.; Lindelöf, B.; Nilsson, G. Increased risk of malignant melanoma in patients with systemic mastocytosis? Acta Derm. Venereol 2014, 94, 583–584.
- Gülen, T.; Möller Westerberg, C.; Lyberg, K.; Ekoff, M.; Kolmert, J.; Bood, J.; Öhd, J.; James, A.; Dahlén, S.E.; Nilsson, G.; et al. Assessment of in vivo mast cell reactivity in patients with systemic mastocytosis. Clin. Exp. Allergy 2017, 47, 909–917.
- Gulen, T.; Hagglund, H.; Dahlen, S.E.; Sander, B.; Dahlén, B.; Nilsson, G. Flushing, fatigue, and recurrent anaphylaxis: A delayed diagnosis of mastocytosis. Lancet 2014, 383, 1608.
- Gulen, T.; Hagglund, H.; Dahlen, B.; Nilsson, G. High prevalence of anaphylaxis in patients with systemic mastocytosis—A single-centre experience. Clin. Exp. Allergy 2014, 44, 121–129.
- Akin, C.; Scott, L.M.; Kocabas, C.N.; Kushnir-Sukhov, N.; Brittain, E.; Noel, P.; Metcalfe, D.D. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood 2007, 110, 2331–2333.
- Sonneck, K.; Florian, S.; Mullauer, L.; Wimazal, F.; Födinger, M.; Sperr, W.R.; Valent, P. Diagnostic and subdiagnostic accumulation of mast cells in the bone marrow of patients with anaphylaxis: Monoclonal mast cell activation syndrome. Int. Arch. Allergy Immunol. 2007, 142, 158–164.
- Kalesnikoff, J.; Galli, S.J. Anaphylaxis: Mechanisms of mast cell activation. Chem. Immunol. Allergy 2010, 95, 45–66.
- Bagos-Estevez, A.G.; Ledford, D.K. Anaphylaxis: Definition, Epidemiology, Diagnostic Challenges, Grading System. Immunol. Allergy Clin. N. Am. 2022, 42, 1–11.
- Wood, R.A.; Camargo, C.A., Jr.; Lieberman, P.; Sampson, H.A.; Schwartz, L.B.; Zitt, M.; Collins, C.; Tringale, M.; Wilkinson, M.; Boyle, J.; et al. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J. Allergy Clin. Immunol. 2014, 133, 461–467.
- Decker, W.W.; Campbell, R.L.; Manivannan, V.; Luke, A.; St Sauver, J.L.; Weaver, A.; Bellolio, M.F.; Bergstralh, E.J.; Stead, L.G.; Li, J.T. The etiology and incidence of anaphylaxis in Rochester, Minnesota: A report from the Rochester Epidemiology Project. J. Allergy Clin. Immunol. 2008, 122, 1161–1165.
- Worm, M. Epidemiology of anaphylaxis. Chem. Immunol. Allergy 2010, 95, 12–21.
- Panesar, S.S.; Javad, S.; de Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The epidemiology of anaphylaxis in Europe: A systematic review. Allergy 2013, 68, 1353–1361.
- Sheikh, A.; Hippisley-Cox, J.; Newton, J.; Fenty, J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J. R. Soc. Med. 2008, 101, 139–143.
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal anaphylaxis: Mortality rate and risk factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178.
- Grabenhenrich, L.B.; Dölle, S.; Moneret-Vautrin, A.; Köhli, A.; Lange, L.; Spindler, T.; Ruëff, F.; Nemat, K.; Maris, I.; Roumpedaki, E.; et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J. Allergy Clin. Immunol. 2016, 137, 1128–1137.e1.
- Worm, M.; Eckermann, O.; Dölle, S.; Aberer, W.; Beyer, K.; Hawranek, T.; Hompes, S.; Koehli, A.; Mahler, V.; Nemat, K.; et al. Triggers and treatment of anaphylaxis: An analysis of 4000 cases from Germany, Austria and Switzerland. Dtsch. Arztebl. Int. 2014, 111, 367–375.
- Sampson, H.A.; Munoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann. Emerg. Med. 2006, 47, 373–380.
- Campbell, R.L.; Hagan, J.B.; Manivannan, V.; Decker, W.W.; Kanthala, A.R.; Bellolio, M.F.; Smith, V.D.; Li, J.T. Evaluation of national institute of allergy and infectious diseases/food allergy and anaphylaxis network criteria for the diagnosis of anaphylaxis in emergency department patients. J. Allergy Clin. Immunol. 2012, 129, 748–752.
- Loprinzi Brauer, C.E.; Motosue, M.S.; Li, J.T.; Hagan, J.B.; Bellolio, M.F.; Lee, S.; Campbell, R.L. Prospective validation of the NIAID/FAAN criteria for emergency department diagnosis of anaphylaxis. J. Allergy Clin. Immunol. Pract. 2016, 4, 1220–1226.
- Baalmann, D.V.; Hagan, J.B.; Li, J.T.; Hess, E.P.; Campbell, R.L. Appropriateness of epinephrine use in ED patients with anaphylaxis. Am. J. Emerg. Med. 2016, 34, 174–179.
- Campbell, R.L.; Li, J.T.; Nicklas, R.A.; Sadosty, A.T.; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: A practice parameter. Ann. Allergy Asthma Immunol. 2014, 113, 599–608.
- Dribin, T.E.; Sampson, H.A.; Camargo, C.A., Jr.; Brousseau, D.C.; Spergel, J.M.; Neuman, M.I.; Shaker, M.; Campbell, R.L.; Michelson, K.A.; Rudders, S.A.; et al. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2020, 146, 1089–1096.
- Dribin, T.E.; Schnadower, D.; Spergel, J.M.; Campbell, R.L.; Shaker, M.; Neuman, M.I.; Michelson, K.A.; Capucilli, P.S.; Camargo, C.A., Jr.; Brousseau, D.C.; et al. Severity grading system for acute allergic reactions: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2021, 148, 173–181.
- Akin, C.; Valent, P.; Metcalfe, D.D. Mast cell activation syndrome: Proposed diagnostic criteria. J. Allergy Clin. Immunol. 2010, 126, 1099–1104.
- Valent, P.; Akin, C.; Bonadonna, P.; Hartmann, K.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Siebenhaar, F.; Sperr, W.R.; Oude Elberink, J.N.G.; et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 1125–1133.
- Gülen, T.; Akin, C.; Bonadonna, P.; Siebenhaar, F.; Broesby-Olsen, S.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, H.N.G.; Butterfield, J.H.; et al. Selecting the right criteria and proper classification to diagnose mast cell activation syndromes: A critical review. J. Allergy Clin. Immunol. Pract. 2021, 9, 3918–3928.
- Schwartz, L.B. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 451–463.
- Valent, P.; Bonadonna, P.; Hartmann, K.; Broesby-Olsen, S.; Brockow, K.; Butterfield, J.H.; Triggiani, M.; Lyons, J.J.; Oude Elberink, J.N.G.; Arock, M.; et al. Why the 20% + 2 Tryptase Formula Is a Diagnostic Gold Standard for Severe Systemic Mast Cell Activation and Mast Cell Activation Syndrome. Int. Arch. Allergy Immunol. 2019, 180, 44–51.
- Butterfield, J.H. Increased Excretion of Mast Cell Mediator Metabolites during Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2023, 11, 2542–2546.
- Gulen, T.; Akin, C. Pharmacotherapy of mast cell disorders. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 295–303.
- Gulen, T. Management of Mediator Symptoms, Allergy, and Anaphylaxis in Mastocytosis. Immunol. Allergy Clin. N. Am. 2023, 43, 681–698.
- Gulen, T.; Akin, C. Idiopathic Anaphylaxis: A Perplexing Diagnostic Challenge for Allergists. Curr. Allergy Asthma Rep. 2021, 21, 11.
More
