Marine actinomycetes produce a multitude of active metabolites, some of which acquire antifouling properties. These antifouling compounds have chemical structures that fall under the terpenoids, polyketides, furanones, and alkaloids chemical groups. These compounds demonstrate eminent antimicrobial vigor associated with antiquorum sensing and antibiofilm potentialities against both Gram-positive and -negative bacteria. They have also constrained larval settlements and the acetylcholinesterase enzyme, suggesting a strong anti-macrofouling activity. Despite their promising in vitro and in vivo biological activities, scaled-up production of natural antifouling agents retrieved from marine actinomycetes remains inapplicable and challenging. This might be attributed to their relatively low yield, the unreliability of in vitro tests, and the need for optimization before scaled-up manufacturing.
- marine actinomycetes
- biofouling
- antibacterial
- antifouling
1. Introduction
2. Fouling
Fouling is a term used to describe the deposition of undesirable organic or inorganic materials on external surfaces. There are two major types of fouling: non-biological and biological fouling (biofouling). Non-biological (also known as inorganic fouling) includes the accumulation of corrosions, oils, salt crystals, and ice on submerged surfaces. Biofouling is the undesirable deposition of organic elements secreted by micro- or macro-organisms (biofilm, EPS, etc.) over submerged surfaces [19][20][21][7,21,22]. As the habitual conditions and causative microorganisms vary, medical, maritime, and industrial fouling forms are very different from one another. While maritime and industrial biofouling are combinations of biofilm and macro- and inorganic fouling, medical biofouling is primarily made up of biofilm buildup [22][23]. Medical fouling may damage indwelling prosthetics, such as fasteners, prosthetic valves, bone plates, dental and orthopedic implants, pacemakers, long-drug-delivery devices, or short-term temporary medical devices, such as catheters, biosensors, ophthalmic lenses, drug-delivery devices, ventilation tubes [23][24]. Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, and Streptococcus viridans are among the organisms that cause infectious biofilm deposition and medical fouling of catheters, tracheal tubes, and ventilators [24][25][25,26]. On the other hand, maritime fouling, which affects ships, sonar devices, pipelines, pillars, offshore infrastructures, oil installations and platforms, undersea cables, etc., is the most prevalent type of environmental fouling [22][23]. After a few seconds from the initial exposure of the submerged surface and the aquatic habitat affluent in nutrients and microorganisms, the multistage process of marine biofouling takes place (Figure 1) [26][38]. Bacteria initially attach themselves to solid surfaces, colonize, and start secreting extracellular polymeric substances (EPSs). Electrostatic and Van der Walls interactions play a substantial role in the early phases of fouling when bacteria cling to the exposed surfaces.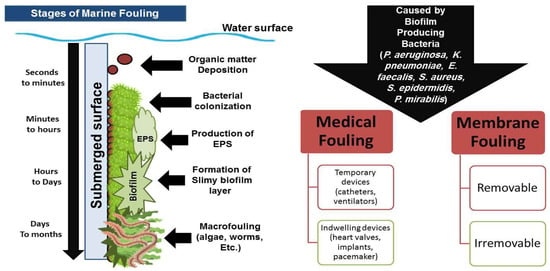
3. Synthetic Antifouling Strategies and Toxicities Associated with Conventional Antifouling Coatings
4. Natural Antifouling Agents (AFs) as an Alternative to Synthetic Antifouling Coatings
Natural AFs are alternatives to synthetic antifouling coatings that are environmentally benign with acceptable compatibilities. Effective fouling prevention without long-lasting negative environmental effects is the key characteristic of an excellent natural antifouling agent [64][83]. One of the primary sources of production for such chemicals is marine microorganisms. AFs function as inhibitors of metabolic signaling pathways, neurotransmitter disruptors, or anti-bioadhesives that eventually block larval settlement (anti-macrofoulers). While neurotransmitter disrupters cause invertebrate larvae to resist settling, anti-bioadhesive AFs work by altering the proteinaceous surface to remove the surface-level inductive hints [17][19]. When a natural AF’s fatal dose (LC50) to minimal concentration inhibiting settling (EC50) ratio (LC50/EC50) is more than 15, it is deemed non-toxic [17][64][19,83]. The terpenoids (terpenes) extracted from the red alga Laurencia rigida prevent the larvae of Amphibalanus amphitrite (also known as Balnus Amphitrite) and Bugula neretina from forming settlements [65][66][84,85]. L. rigida extracts are rich in terpenoids, elatol, and deschloroelatol; anti-settlement activity against the barnacle larvae was tested using cyprids of Amphibalanus amphitrite (also known as B. amphitirite) against three synthetic antifouling agents, irgarol 1051, Sea-Nine 211, and nopcocide N-96. Both terpenoids inhibited the attachment of B. amphitirite cypris larvae at a conc. of 10 ng cm−2 with etalol inhibiting 100% of larval settlement and deschloroelatol exerting 90% inhibition. The control synthetic antifouling agents were, however, less effective than elatol and deschloroelatol. Irgarol 1051 was the least active compound at 10 ng cm−2. It is interesting to highlight the eminent lethality of both elatol and deschloroelatol to B. amphitrite nauplii (early larval stages), incurring 100% mortality at 100 ng cm−2 and almost 90% and 50–60% mortality at 10 and 1 ng cm−2, respectively. Halogenated terpene derivatives are also useful natural AFs in the prevention of larval adhesion. Sargassum tenerrimum phlorotannins hinder the metamorphosis of Hydroides elegans [67][86]. S. tenerrimum extract is opulent with phlorotanins, phloroglucinol, and tannic acid. The compounds elucidate variable anti-adhesive potentialities against Hydroides elegans larva settlement. Phlorotanins attain high safety profiles since the reported LC50 score was 27 times higher than their EC50 score, which were 13.984 ppm and 0.526 ppm, respectively. This gives an LC50/EC50 score of 26 for S. tenerrimum phlorotanins. Phloroglucinol reports an EC50 score of 5.231 ppm and an LC50 score of 206.823 ppm (LC50/EC50 ratio of 38). At non-toxic doses, the gorgonian Junceella juncea diterpene extract exhibits potent antifouling activity against A. amphitrite larval colonization [68][88]. The J. juncea extract rich with briarane diterpene, including juncin ZII, attained moderate insecticidal and antifeedant activity against Spodoptera litura second-instar larvae when compared to synthetic material, azadirachtin. When tested against Balanus amphitrite settlement, juncin ZII exerted significant anti-settlement effects with an EC50 score of 0.004 μg/mL [68][88]. The brown seaweed Canistrocarpus cervicornis’ dolastane, seco-dolastane diterpene, and isolinearol affluent extract hinder Perna perna mussel settlement [69][89]. The Streptomyces tumemacerans albofungin is a potent antifouling agent with an equivalent efficacy to butenolide against Amphibalanus amphitrite larvae and an EC50 score of 1.6 μg/mL. It also acquires acceptable safety profiles with an LC50/EC50 ratio > 100 even when used at high concentrations (up to 40 μg/mL) [70][92]. The anti-adhesive characteristics of the previously mentioned natural antifouling agents may be assigned to the inhibition of the phenoloxidase and tyrosinase enzymes that regulate the crosslinking and creation of the adhesive plaques needed to anchor the mussels’ byssal and substrata. This was observed when arctic marine sponge Stryphnus fortis bromotyrosine-rich extract blocked blue mussel phenoloxidase and hindered their settlement [71][93]. The first known antifouling benzenoid, 3-chloro-2,5-dihydroxybenzyl alcohol, retrieved from Ampelomyces sp. UST040128, is known for its anti-larval settlement effect against both Balanus amphitrite cyprids and Hydroides elegans larvae. Its EC50 score for B. amphitrite ranged from 3.19 μg/mL to 3.81 μg/mL, and the LC50 score was 266.8 μg/mL. Upon testing on Hydroides elegans, the observed effect was dose-dependent, and the EC50 score was 0.67 μg/mL to 0.78 μg/mL, and the LC50 value was 2.64 μg/mL [72][94]. Amibromdole isolated from Sarcophyton sp. fungus is a halogenated benzenoid with powerful antifouling activity against Balnus amphitrite. Similarly, pestalachlorides E and F secreted by the fungal strain Pestalotiopsis ZJ-2009-7-6 elucidates strong antifouling activity against B. amphitrite larval settlement [16][18]. Both compounds have EC50 scores ranging from 1.65 to 0.55 μg mL−1 against the barnacle Balanus amphitrite’s larval settlement with an LC50/EC50 score > 15, suggesting their safety and efficacy [73][95]. Dihydroquinolin-2-one-containing alkaloids isolated from Scopulariopsis sp. fungal extracts are known for their eminent anti-macrofouling vigor against Balanus amphitrite larval colonization with acceptable safety and therapeutic profiles [74][96]. The dihydroquinolin-2-ones have average EC50 scores of ~25–50 μg/mL and LC50 scores of ~3.79–7.85 μM when tested against the brine shrimp A. salina [74][96]. They are also known for their bactericidal effects against the fouling bacterial species S. aureus, B. cereus, V. parahaemolyticus, N. brasiliensis, and P. putida, with MIC scores of 0.78, 1.56, 6.25, 0.78, and 1.56 μM, respectively [74][96]. The N-methyltetrahydroellipticine and furoquinoline alkaloids, kokusaginine and flindersiamine, purified from Atlantic yellow guatambu’ Aspidosperma australe and white guatambu’ Balfourodendron riedelianum trees elucidate eminent anti-macrofouling potential against the Mytilus edulis platensis mussel [75][98]. A. australe bark extract was opulent with pyridocarbazole olivacine, indole alkaloids uleine, and N-methyltetrahydroellipticine, where N-methyltetrahydroellipticine yielded the best anti-adhesive macrofouling activity with an EC50 value of 1.56 nmol cm−2 against Mytilus edulis platensis mussels. Second to N-methyltetrahydroellipticine in its anti-macrofouling vigor is the kokusaginine retrieved from B. riedelianum bark extract that scored an EC50 value of 3.86 nmol cm−2. The others, flindersiamine, olivacine, and uleine, recorded EC50 scores of 5.56, 7.59, and 9.95 nmol cm−2, respectively.5. Marine Actinomycetes as Sources of Natural Antifouling Agents
Actinomycetes, in particular, marine actinomyctes, are highly important industrial sources of secondary metabolites that include a wide range of antimicrobial, antibacterial, and antifouling agents. They belong to the Gram-positive bacterial order Actinomycetales and display a wide range of distinctive features, such as habitat, ideal pH, thermophilicity, and moisture tolerance. They interact with a wide range of aquatic animals, including invertebrates, like sponges, corals, and echinoderms, as well as vertebrates, like pufferfish corals, and a variety of invertebrates [76][77][130,131]. The evolution of secondary metabolic pathways may be influenced by these interactions, which may promote particular chemical ecologies. Although most strains have been identified from sediments, marine actinomycetes can coexist with other species and live in both planktonic and biofilm habitats [13][15].
